Pod therapy for type 2 diabetes

Experience the peace of mind of getting your insulin without the disruption and inconvenience of daily injections.
Real People, Real Success
Meet Milli, a nurse, caregiver and proud grandmother of 6 and living with type 2 diabetes. Watch how Omnipod 5 has simplified her life.
Results That Matter
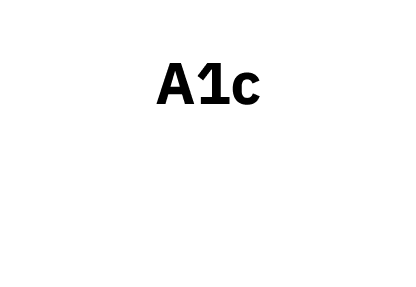
For people with type 2 diabetes, Omnipod 5 lowered A1c by 2.1% for people with an A1c of 9% or more.*
For people with type 2 diabetes, Omnipod 5 improved time in range by nearly 5 hours per day.*
Omnipod Solutions For Adults with Type 2 Diabetes


Omnipod 5
Tubeless automated insulin delivery, now cleared for adults with type 2 diabetes, ages 18+. Omnipod 5 uses your CGM sensor value and trend to automatically increase, decrease, or pause insulin delivery every 5 minutes.
Omnipod DASH
Another waterproof tubeless insulin delivery option available to people with insulin requiring type 2 diabetes. Omnipod DASH includes the Personal Diabetes Manager (PDM) and the Pod, but does not integrate with a CGM sensor.
The Power of the Pod
- Small & easily hidden
- Tubeless
- Waterproof†
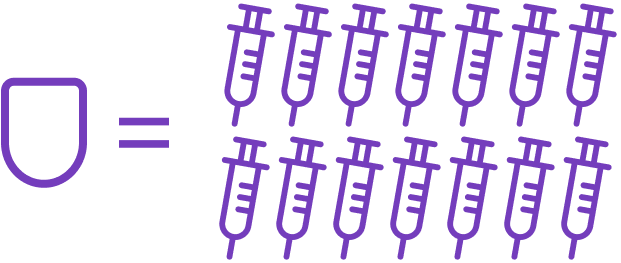
- 1 Pod replaces up to 14 injections*
Get Started Today
Interested in a complimentary benefits check? Click below to learn more.
Affordable solutions
On average the copay for Omnipod is less than $50 per month‡
Omnipod is available for pick up at your favorite pharmacy. No upfront costs, just pay as you go. Even better news, Omnipod is covered under Medicare Part D.
“With Omnipod 5 not only did my A1C improve, but I no longer had the big swings in my levels like I did when I was on shots.”
Milli
Living with type 2 diabetes
Omnipod® 5 user
Pods, Pumps and
Type 2 Diabetes
Pod therapy is different in many ways to traditional insulin pumps. Learn more about why Omnipod is a true game changer for people with type 2 diabetes.
†The Pod has a waterproof IP28 rating for up to 25 feet for 60 minutes. The PDM/Controller is not waterproof.
‡Omnipod DASH average calculation based on a consumption of ten (10) Pods per month. Majority defined as at least 70% of patient co-pays under $50 per month. 276,024 paid claims between January 1st, 2021 and December 31st, 2021, both for commercial plans and Medicare, were analyzed. Actual co-pay amount depends on patient’s health plan and coverage, they may fluctuate and be higher or lower than the advertised amount on a monthly basis. Source: Data on file.
Calculated based on a consumption of ten (10) Pods per month. Majority defined as at least 70% of patient co-pays $50 or less per month . Among All Paid Omnipod 5 G6 Pods Commercial and Medicare Claims from August 2022 through July 2023. Includes benefits and offerings available through Insulet, such as the copay card program. Actual co-pay amount depends on patient’s health plan and coverage, they may fluctuate and be higher or lower than the advertised amount on a monthly basis. Source: IQVIA OPC Library
Risk Statement
The Omnipod DASH Insulin Management System is indicated for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin.
The Omnipod 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and type 2 diabetes mellitus in persons 18 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoRapid®, Humalog®, and Admelog®.The Omnipod 5 Pump (Pod) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The Omnipod 5 Pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. SmartAdjust™ technology is intended for use with compatible integrated continuous glucose monitors (iCGM) and to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values. The Omnipod 5 SmartBolus Calculator is intended to calculate a suggested bolus dose based on user-entered carbohydrates, most recent sensor glucose value (or blood glucose reading if using fingerstick), rate of change of the sensor glucose (if applicable), insulin on board (IOB), and programmable correction factor, insulin to carbohydrate ratio, and target glucose value.
WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.The Omnipod 5 System is NOT recommended for people who are unable to monitor glucose as recommended by their healthcare provider, are unable to maintain contact with their healthcare provider, are unable to use the Omnipod 5 System according to instructions, are taking hydroxyurea as it could lead to falsely elevated CGM values and result in over-delivery of insulin that can lead to severe hypoglycemia, and do NOT have adequate hearing and/or vision to allow recognition of all functions of the Omnipod 5 System, including alerts, alarms, and reminders. Device components including the Pod, CGM transmitter, and CGM sensor must be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment. In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to MRI, CT, or diathermy treatment can damage the components. Visit www.omnipod.com/safety for additional important safety information.
WARNING: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia.