What is Omnipod?
Omnipod® gives you more with less
Omnipod provides continuous insulin delivery through a tubeless, waterproof* insulin pump called a Pod—all with no multiple daily injections. Get up to 3 days (72 hours) of continuous insulin delivery and freedom with these innovative features:
- Wearable: Place the Pod almost anywhere you’d normally inject
- Waterproof*: Take your insulin anywhere life takes you, even while swimming
- Tangle-proof: Forget the tubes of traditional insulin pumps
How Omnipod Works
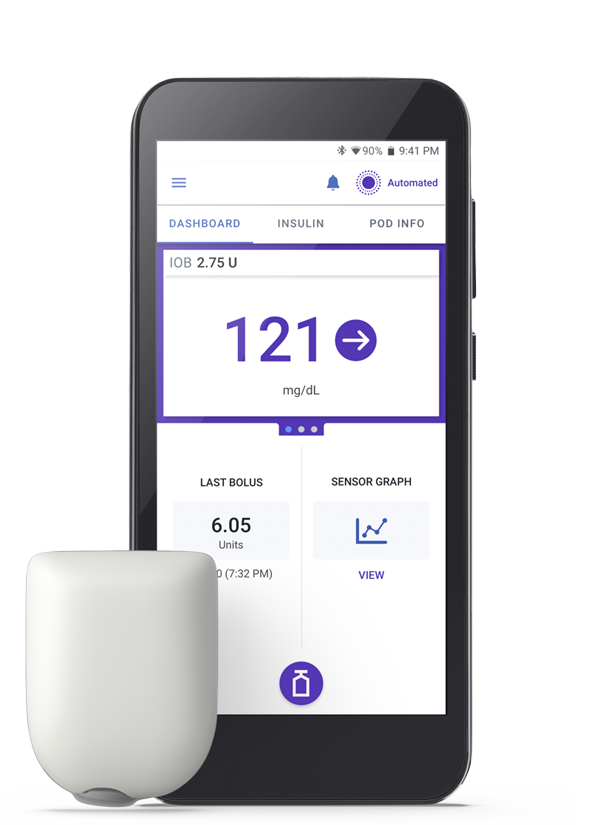
Omnipod tubeless systems are made of two main components, the Pod and a smartphone-like PDM/Controller. The Pod is a small device that you fill with insulin and wear directly on your body. The Pod receives insulin delivery instructions from the Controller. It then delivers insulin into your body through a small, flexible tube called a cannula.
#1 Prescribed Automated Insulin Delivery System1
1USA 2023, Data on file

COMPATIBLE WITH | ||
Image

| Image

| Image

|
Omnipod® 5
With Omnipod 5 Automated Insulin Delivery System, your diabetes devices are in sync without tubes to tie you down. It’s everything you love about Omnipod—waterproof†, wearable, tubeless—and so much more:
- SmartAdjust™ technology automatically increases, decreases, or pauses insulin every 5 minutes, based on your customized target—helping to protect against highs and lows2-4
- Activity feature when enabled, reduces insulin delivery when glucose typically goes low, like when exercising
- SmartBolus Calculator, the only AID system with a built-in bolus calculator that automatically incorporates your CGM value and trend, so you don’t have to
- Compatible with Dexcom G6, Dexcom G7 and the FreeStyle Libre 2 Plus Sensor.
You will still need to bolus for meals. With Omnipod 5, this is done with the Omnipod 5 App or Controller.
†The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Omnipod 5 Controller is not waterproof. The Dexcom G6, when properly installed and G7 CGMs have an IP28 rating for up to 8 feet for 24 hours. The FreeStyle Libre 2 Plus Sensor is water-resistant in up to 1 meter (3 feet) of water. Do not immerse longer than 30 minutes.
Omnipod DASH®
A tubeless, wireless insulin management system that lets you experience more freedom with fewer daily hassles. Wear the Pod for up to 3 days (72 hours) of continuous insulin delivery, without multiple daily injections. And the convenience doesn’t stop there, get it all through the pharmacy. Even the Personal Diabetes Manager (PDM) comes at no charge to you‡.
Omnipod DASH is cleared for anyone with insulin requiring diabetes.


A 37-year road to freedom
Clare was diagnosed with type 1 diabetes over 40 years ago. After decades of multiple daily injections, blood glucose swings, and dangerous lows, Omnipod helped her simplify life with diabetes.
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
Like what you see?
Now that you’ve learned a little about Omnipod, you might be ready to take the next step. To get more information on whether Omnipod is right for you, connect with an Omnipod Specialist for more information. Or, get started with an Omnipod DASH or Omnipod 5 trial§.
1. USA 2023, Data on file
2. Brown S. et al. Diabetes Care (2021). Study in 240 people with T1D aged 6 - 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in adults/adolescents and children, standard therapy vs 3-month Omnipod 5: 32.4% vs. 24.7%; 45.3% vs. 30.2%. Average time with low blood glucose in adults/adolescents and children, standard therapy vs 3-month Omnipod 5: 2.9% vs. 1.3%; 2.2% vs. 1.8%. Study funded by Insulet.
3. Sherr JL, et al. Diabetes Care (2022). Study in 80 people with T1D aged 2 - 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 39.4% vs. 29.5%. Average time with low blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 3.43% vs. 2.46%. Study funded by Insulet.
4. Pasquel FJ, et al. Presented at: ADA; June 21-24, 2024; Orlando, FL. Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time <70 mg/dL as measured by CGM: ST = 0.2%, 3-mo Omnipod 5 = 0.2%, P<001. Mean time >180 mg/dL as measured by CGM: ST = 54%, 3-mo Omnipod 5 = 34%, P<0.001. Comparison is relative change.
§Omnipod DASH® 30-Day Free Trial
Terms and Conditions
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod DASH 30-day Free Trial Program (the “Program”) is open to patients who have a valid Omnipod DASH prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. The Program is open only to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod® 5, Omnipod DASH or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod DASH prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs, or where prohibited by law. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a 30-day supply of Omnipod DASH products at no cost for them. Eligible participants have two (2) options, based on the following:
• A participant shall sign the 30-day Omnipod DASH Free Trial Acknowledgement through the appropriate platform provided by Insulet’s pharmacy partner.
• Once Insulet, or its pharmacy partner, has received the request, including a valid prescription for the Omnipod DASH Intro Kit and Omnipod DASH Pods, the participant’s benefits will be checked by Insulet or its pharmacy partner.
• If the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet, or its pharmacy partner, shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod DASH 30-day initial shipment, in accordance with Section 3, below.
• If the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod DASH Intro Kit, in accordance with Section 4, below.
• For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
• Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod DASH Copay Card, participant shall receive electronically one (1) Omnipod DASH Copay Card, valid for a single use, in the amount required for the participant to procure a 30-day supply of Omnipod DASH Pods at a participating pharmacy. In addition, Insulet’s pharmacy partners shall ship, at no cost to participant, one (1) Omnipod DASH Starter Kit, which shall include:
• One (1) Omnipod DASH Personal Diabetes Manager (PDM)
• One (1) Omnipod DASH User Guide
• One (1) PDM charging cable
The Omnipod DASH Starter Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Starter Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Starter Kit and to verify the content of the Starter Kit.
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod DASH Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod DASH Intro Kit, which shall include:
• One (1) Omnipod DASH PDM
• Eleven (11) Omnipod DASH Pods
• One (1) Omnipod DASH User Guide
• One (1) PDM charging cable
The Omnipod DASH Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.
§Omnipod® 5 Intro Kit 30-Day Trial
Terms and Conditions
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod 5 Intro Kit 30-day trial program (the “Program”) is open to patients who have a valid Omnipod 5 prescription as well as a compatible CGM prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. In order to be eligible, the patient’s eligible insurance plan must include coverage for Omnipod 5 Pods. The Program is open to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod 5, Omnipod DASH® or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod 5 or compatible CGM prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs. It is not valid for cash-paying participants or where prohibited by law. A participant is considered cash-paying where the participant has no insurance coverage for Omnipod 5 or where the participant has commercial or private insurance but Insulet determines in its sole discretion the participant is effectively uninsured because such coverage does not provide a material level of financial assistance for the cost of an Omnipod 5 prescription. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Participants receiving their products through the Durable Medical Equipment or Pharmacy Durable Medical Equipment channel are not eligible to participate in the copay card program. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a limited supply of Omnipod 5 products at no cost for them. Eligible participants have two (2) options, based on the following:
• A participant shall sign the Omnipod® 5 Intro Kit 30-Day Trial Acknowledgement through the appropriate platform provided by Insulet.
• Once Insulet has received the request, the request shall be escalated to Insulet’s pharmacy partner, where a request for a prescription shall be sent to the participant’s healthcare professional. If a valid prescription is received, both for the Omnipod 5 Intro Kit and the Omnipod 5 Pods, the participant’s benefits will be checked by Insulet or its partners.
• IF the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod 5 Intro Kit, in accordance with Section 3, below.
• IF the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod 5 Intro Kit, in accordance with Section 4, below.
• For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
• Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
• In order to use the Omnipod 5 System in Automated Mode, the User must also procure a compatible CGM. This program does not include supply of a compatible CGM.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod 5 Copay Card, participant shall receive electronically one (1) Omnipod 5 Copay Card, valid for a single use, in the amount required for the participant to procure one (1) Omnipod 5 Intro Kit, which shall include:
• One (1) Omnipod 5 Controller
• Ten (10) Omnipod 5 Pods
• One (1) Omnipod 5 User Guide
• One (1) Controller charging cable
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod 5 Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod 5 Intro Kit, which shall include:
• One (1) Omnipod 5 Controller
• Ten (10) Omnipod 5 Pods
• One (1) Omnipod 5 User Guide
• One (1) Controller charging cable
The Omnipod 5 Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.