Take control of your Type 1 diabetes.
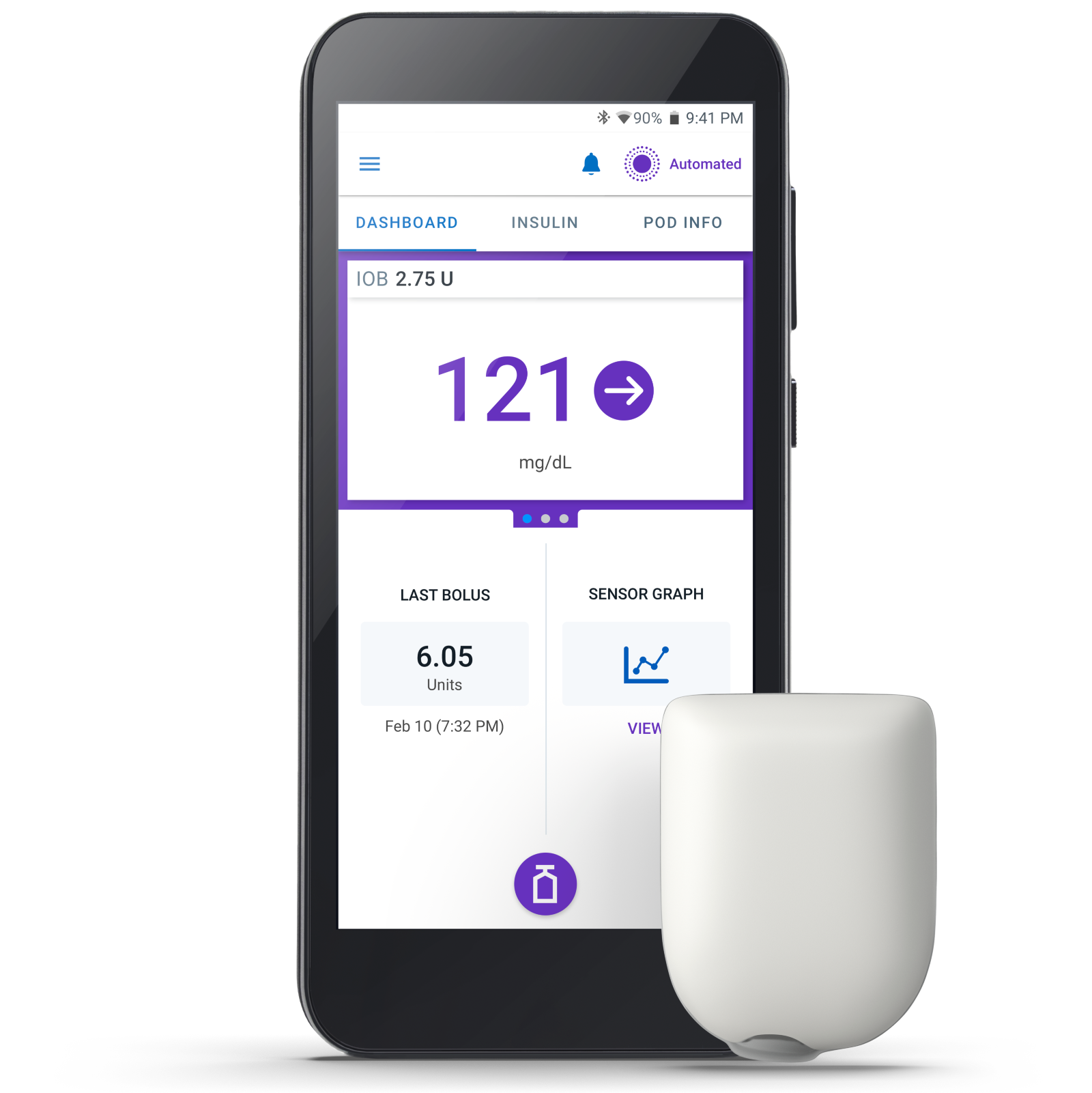

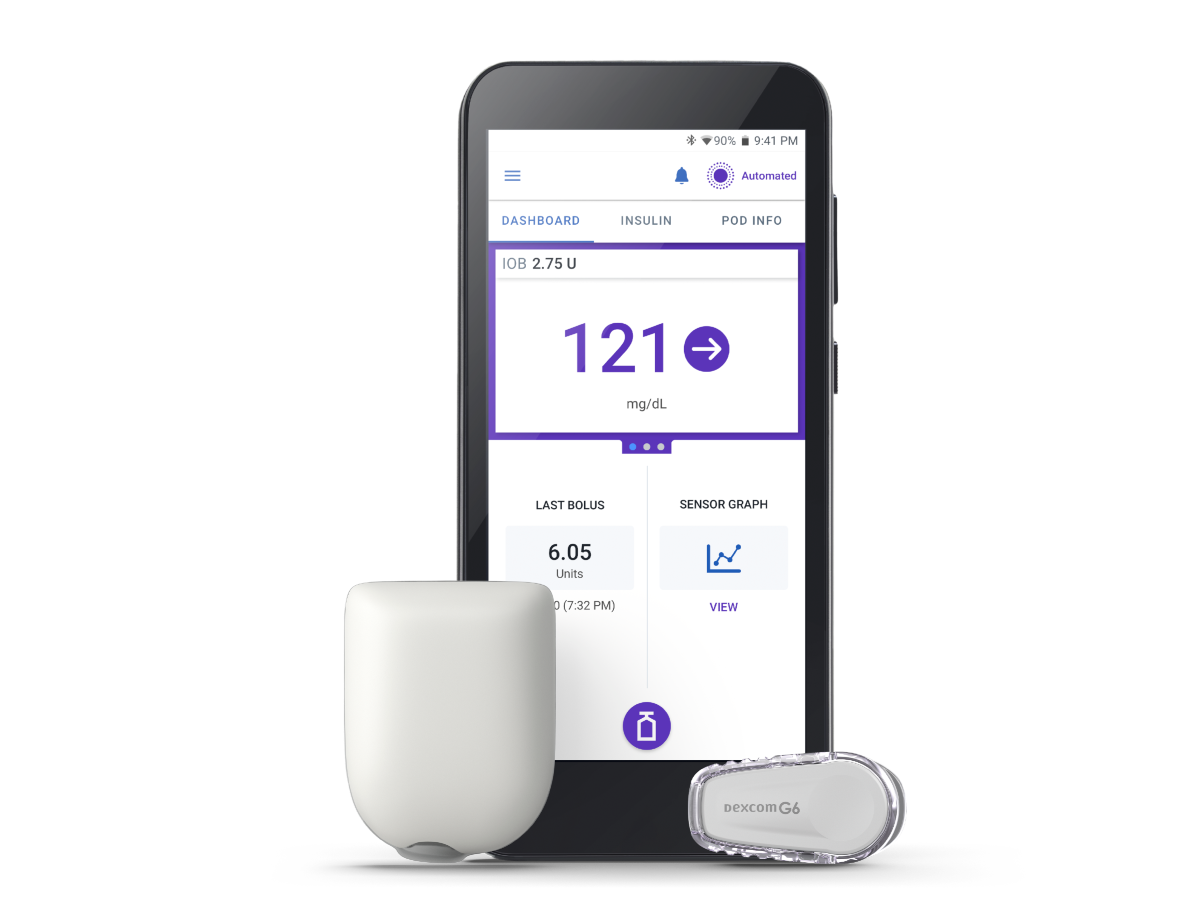
Omnipod® 5: A tube-free automated insulin delivery system for type 1 diabetes
Type 1 diabetes doesn’t take a break. But managing your insulin therapy with Omnipod 5 can give you one. Omnipod 5 is different from traditional insulin pumps—it’s tube-free and waterproof*, and can be worn almost anywhere you would give an insulin injection.
Plus, you can control it all using the Omnipod 5 App on the Controller or a compatible smartphone, making giving insulin look like sending a text.
Say goodbye to multiple daily injections and say hello to Omnipod 5.
Simplify your everyday life
Whether you’re a parent or caregiver of a child with type 1 diabetes or living with type 1 diabetes yourself, the innovative, wearable Omnipod insulin therapy can help lighten the load of day-to-day diabetes management. Omnipod 5 users all over the world experience the benefits of this tubeless insulin delivery system every day.
Unique benefits of Omnipod include:
- Eliminates the need for multiple daily injections—Each Pod delivers insulin continuously for 3 consecutive days (up to 72 hours)
- Tangle-proof, waterproof*, and durable—Ideal for the physical demands of day-to-day life
- Tubeless— Walk freely through every open door, leaving doorknobs in the dust. No belts, fanny packs, or pockets required
- Easy to use—The Pod is easy to apply, insertion is quick and virtually painless, and the Controller offers the flexibility to manage your insulin delivery in the palm of your hand
- Lifestyle compatible—The Pod is small enough to be worn discreetly under clothing, almost anywhere you’d give yourself an injection, and the controller is small enough to fit in a purse or pocket. Plus, Omnipod 5 can even be controlled from a compatible smartphone.


How does an automated insulin delivery system like Omnipod 5 help?
Omnipod 5 works with a compatible CGM sensor to help keep glucose levels in range. The System increases, decreases, or pauses insulin delivery every 5 minutes based on glucose values and trend to help protect against highs and lows, day and night.1,2
Learn more about how automated insulin delivery can help with your diabetes management
Omnipod 5 is covered by most insurance plans, including Medicare Part D
Omnipod 5 has over 90% insurance coverage‡, and unlike traditional pumps, is available at the pharmacy.
Omnipod 5 is also covered by Medicare Part D and is the only pump eligible by Medicare for people with Type 1 diabetes.


Check your coverage
Choose your preferred path to answer a few questions. Once complete, a member of the Omnipod Pharmacy support team** will reach out with your expected copay and free trial† eligibility.
I’d like to check my coverage
Find out your estimated copay
I'd like a free trial of Omnipod†
Take the Pod for a test drive
Risk Statement
The Omnipod 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and individuals with type 2 diabetes ages 18 years and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoRapid®, Humalog®, and Admelog®.The Omnipod 5 Pump (Pod) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The Omnipod 5 Pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. SmartAdjust™ technology is intended for use with compatible integrated continuous glucose monitors (iCGM) and to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values. The Omnipod 5 SmartBolus Calculator is intended to calculate a suggested bolus dose based on user-entered carbohydrates, most recent sensor glucose value (or blood glucose reading if using fingerstick), rate of change of the sensor glucose (if applicable), insulin on board (IOB), and programmable correction factor, insulin to carbohydrate ratio, and target glucose value.
WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.The Omnipod 5 System is NOT recommended for people who are unable to monitor glucose as recommended by their healthcare provider, are unable to maintain contact with their healthcare provider, are unable to use the Omnipod 5 System according to instructions, are taking hydroxyurea as it could lead to falsely elevated CGM values and result in over-delivery of insulin that can lead to severe hypoglycemia, and do NOT have adequate hearing and/or vision to allow recognition of all functions of the Omnipod 5 System, including alerts, alarms, and reminders. Device components including the Pod, CGM transmitter, and CGM sensor must be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment. In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to MRI, CT, or diathermy treatment can damage the components. Visit www.omnipod.com/safety for additional important safety information.
WARNING: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia.
*The Pod has an IP28 rating for up to 25 feet for 60 minutes. The PDM is not waterproof.
‡Reflects coverage for Omnipod 5 G6 Intro Kit and Omnipod 5 G6 Pods. Source: Insulet Data on File As of June 1 2023.
**ASPN Pharmacies and its dedicated staff power the Omnipod Pharmacy Support Program. The ASPN team is here for you to check your coverage and help coordinate fulfillment at your pharmacy of choice.
1. Study in 240 people with T1D aged 6 - 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average overnight time (12AM-6AM) with high blood glucose in adults/adolescents and children for standard therapy vs. Omnipod 5 = 32.1% vs. 20.7%; 42.2% vs. 20.7%. Average day time (6AM-12AM) with high blood glucose in adults/adolescents and children for standard therapy vs. Omnipod 5 = 32.6% vs. 26.1%; 46.4% vs. 33.4%. Median overnight time (12AM-6AM) with low blood glucose in adults/adolescents and children for standard therapy vs. Omnipod 5 = 2.07% vs. 0.82%; 0.78% vs. 0.78%. Median day time (6AM-12AM) with low blood glucose in adults/adolescents and children for standard therapy vs. Omnipod 5 = 1.91% vs. 1.08%; 1.17% vs. 1.62%. Brown et al. Diabetes Care (2021).
2. Study in 80 people with T1D aged 2 - 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose overnight from CGM in standard therapy vs. Omnipod 5 = 38.4% vs. 16.9%. Average time with high blood during daytime from CGM in standard therapy vs. Omnipod 5 = 39.7% vs. 33.7%. Average time with low blood glucose overnight from CGM in standard therapy vs. Omnipod 5 = 3.41% vs. 2.13%. Average time with low blood glucose during daytime from CGM in standard therapy vs. Omnipod 5 = 3.44% vs. 2.57%. Sherr JL, et al. Diabetes Care (2022).
Omnipod® 5 Intro Kit 30-Day Trial
Terms and Conditions
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod 5 Intro Kit 30-day trial program (the “Program”) is open to patients who have a valid Omnipod 5 prescription as well as a compatible CGM prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. In order to be eligible, the patient’s eligible insurance plan must include coverage for Omnipod 5 Pods. The Program is open to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod 5, Omnipod DASH® or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod 5 or compatible CGM prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs. It is not valid for cash-paying participants or where prohibited by law. A participant is considered cash-paying where the participant has no insurance coverage for Omnipod 5 or where the participant has commercial or private insurance but Insulet determines in its sole discretion the participant is effectively uninsured because such coverage does not provide a material level of financial assistance for the cost of an Omnipod 5 prescription. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Participants receiving their products through the Durable Medical Equipment or Pharmacy Durable Medical Equipment channel are not eligible to participate in the copay card program. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a limited supply of Omnipod 5 products at no cost for them. Eligible participants have two (2) options, based on the following:
- A participant shall sign the Omnipod® 5 Intro Kit 30-Day Trial Acknowledgement through the appropriate platform provided by Insulet.
- Once Insulet has received the request, the request shall be escalated to Insulet’s pharmacy partner, where a request for a prescription shall be sent to the participant’s healthcare professional. If a valid prescription is received, both for the Omnipod 5 Intro Kit and the Omnipod 5 Pods, the participant’s benefits will be checked by Insulet or its partners.
a) IF the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod 5 Intro Kit, in accordance with Section 3, below.
b) IF the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod 5 Intro Kit, in accordance with Section 4, below.
c) For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
d) Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
e) In order to use the Omnipod 5 System in Automated Mode, the User must also procure a compatible CGM. This program does not include supply of a compatible CGM.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod 5 Copay Card, participant shall receive electronically one (1) Omnipod 5 Copay Card, valid for a single use, in the amount required for the participant to procure one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod 5 Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
- One (1) Controller charging cable
The Omnipod 5 Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.