How the Omnipod DASH® System may help improve glycaemic control1
Meeting recommended targets and day-to-day insulin management is a burden that many people living with T1D know all too well. Consisting of a lightweight*, smartphone-like Personal Diabetes Manager (PDM) and wearable tubeless Pod2, the Omnipod DASH® System has been associated with a reduction in acute complications3, and has been reported to improve Quality of Life, provide a lifestyle benefit and simplify diabetes management1.
The majority of people with T1D do not meet their HbA1c targets4
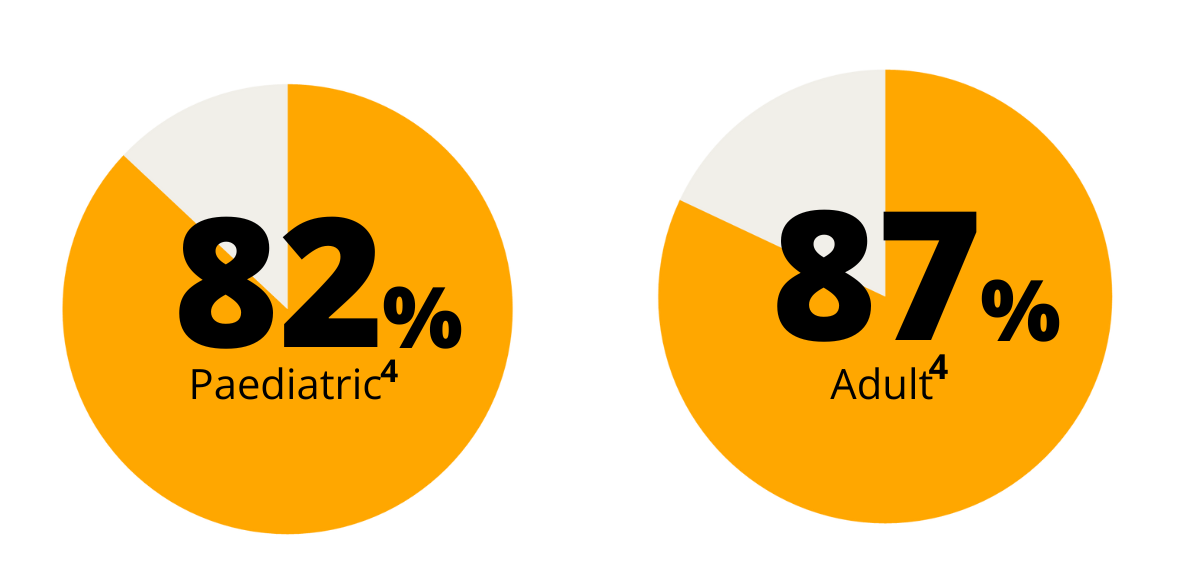

In a cross-sectional analysis of 7,988 individuals with type 1 diabetes included in a Australian diabetes registry, 82% of the paediatric cohort (< 18 years) and 87% of the adult cohort were not meeting the international glycaemic target of HbA1c <53 mmol/mol (7.0%)4.
Can simplifying your patients' insulin management help them with their diabetes?
In a real world study of 241 Australians living with type 1 diabetes using Omnipod DASH®, users experienced a reduction in HbA1c of 0.5% after three months and 85% reported simplified diabetes management1.
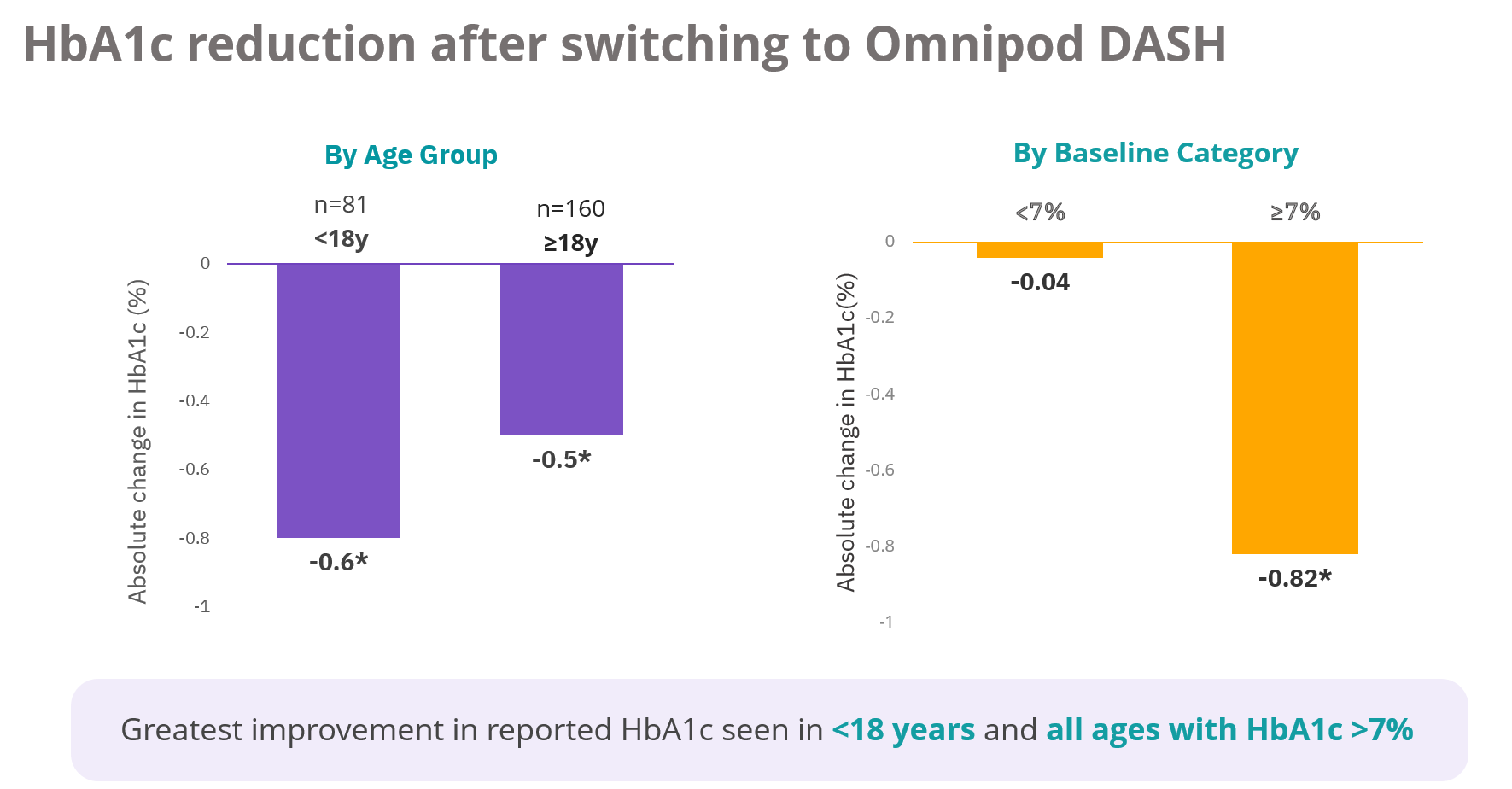

Study description
HbA1c alone does not provide a measure of glycaemic variability or hypoglycaemia5
Even for those people living with T1D who do meet their target HbA1c, their Time in Range (TIR)* may fluctuate below and above glucose range, increasing their risk of complications and reducing their Quality of Life6,7.
Children, adolescents and adults who switched from MDI to the Omnipod DASH® System experienced a clinically significant improvement in HbA1c of -1.0%8*
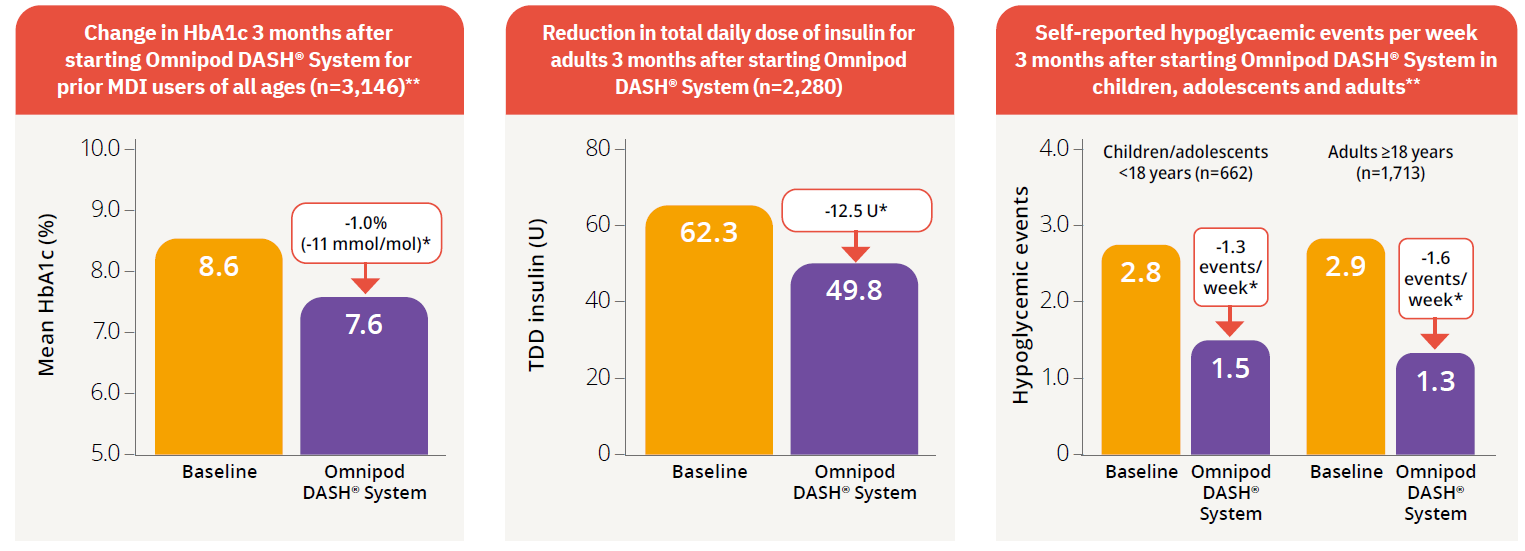

Study description
See The Omnipod DASH® Data
Apart from being associated with an improvement in glycaemic control, Omnipod® has also been associated with a reduction in acute complications3 for patients.
Arrange a meeting with an Omnipod® representative
Do you have questions about the Omnipod DASH® System? Our team is on call to provide you with the information you need and help you to determine which of your patients could best benefit from the Omnipod DASH® System.
Be the first to know
Stay up to date and be in the know when it comes to all things Insulet. By signing up for our mailing list, you will be in the know when it comes to training and events, Omnipod® published data and first-hand experiences from Podders®.
IMPORTANT SAFETY INFORMATION: The Omnipod DASH®Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. The Omnipod DASH® System has been tested and found to be safe for use with the following U-100 insulin: Novolog®, Humalog®, Apidra®Fiasp®or Admelog®. Refer to the Omnipod DASH® Insulin Management System User Guide for complete safety information including indications, contraindications, warnings, cautions, and instructions.
References: 1. Nash B, et al. Real-world outcomes following initiation of the Omnipod DASH tubeless insulin pump for people with type 1 diabetes (T1D) in Australia Poster ATTD 2024 E poster EV338 / #502. 2. Omnipod DASH® Insulin Management System [User Guide]. 2019. 3. Biester T, et al. Diabetes Technol Ther. 2021 4. Holmes-Walker J et al, Internal Medicine 2021. Outcomes in Australasian children and adults with type 1 diabetes: failure to meet targets across the age spectrum. doi:10.1111/imj.15426 5. ADA. Diabetes Care, January 2021. 6. Battelino T, et al. Diabetes Care. 2019;42(8):1593-1603 7. Beck RW, et al. Lancet Diabetes Endocrinol. 2017;5(9):700-708. 8. Aleppo G, et al. Improvements in Glycemic Outcomes in 4738 Children, Adolescents, and Adults with Type 1 Diabetes Initiating a tubeless Insulin Management System.
Diabetes Therapy 2023; 14: 593-610.