Omnipod® 5 Automated Insulin Delivery System
The Omnipod® 5 System will be Australia’s first TGA approved tubeless, automated and waterproof* insulin delivery system, for people with type 1 diabetes aged 2 years and older. Automated insulin delivery is available when used with a compatible Continuous Glucose Monitor (CGM). Compatible sensor information will be provided closer to launch. The exclusive SmartAdjust™ technology automatically adjusts your basal insulin every five minutes based on your CGM readings, helping to protect you against highs and lows by improving time in range, day and night1,2.
Omnipod 5 will be available for your patients in early 2025. Learn more about our OmnipodPromise®, a pathway for your patients (who currently access Omnipod DASH via private health insurance) who wish to transition to Omnipod 5 once it becomes available.
The System - 3 Simple Parts
Controller + Pod + Sensor


Omnipod 5 Controller
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod’s operations using Bluetooth® wireless technology.
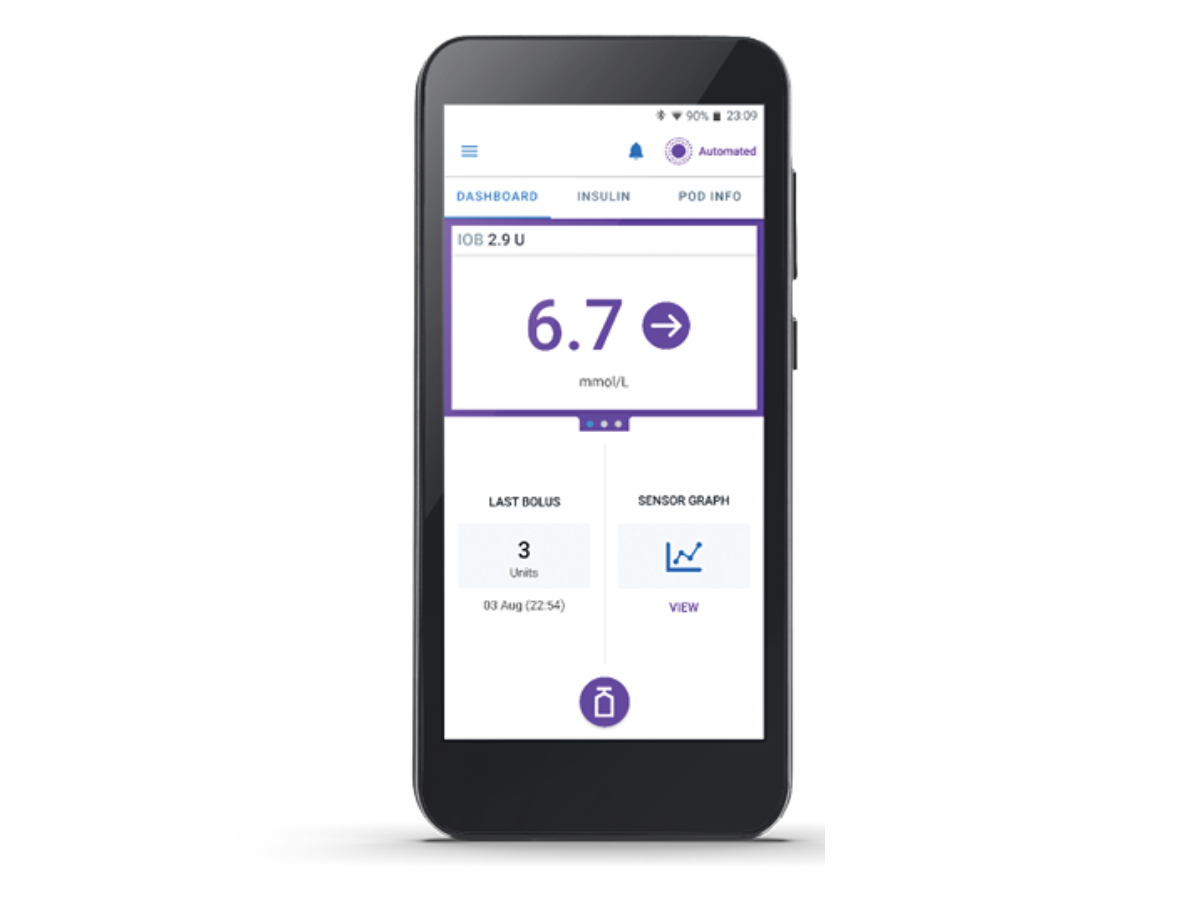



Pod
Tubeless, wearable, and waterproof*, the Pod, with built-in SmartAdjust™ technology, sits on your patient’s body and automatically adjusts insulin delivery for up to 3 days or 72 hours.
Sensor
Continuously sends glucose values to the Pod, so your patients can get real-time data without the finger pricks† . The Pod and sensor need to be in ‘line of sight’ to stay in Automated Mode. Placing them on the same side of the body allows for best communication between the devices.


SmartAdjust ™ Technology
Predicts
Glucose 60 minutes into the future
Adjusts
Insulin delivery using the selected glucose target
Delivers
Insulin doses every 5 minutes (as needed)
Always adjusting, so you don’t have to.
Omnipod® 5 with SmartAdjust™ technology automatically adjusts to your patients’ personal needs by increasing, decreasing, or pausing insulin delivery every five minutes – which may help prevent highs and lows.1,2
Learn how SmartAdjust™ works and tips for initialisation with your patients.
Stay in the know!
Sign up here to get the latest news and updates.
Find answers to the most frequently asked questions on Omnipod® 5
What is the Omnipod 5 System?
How does the Omnipod 5 System differ from the Omnipod DASH System?
When will Omnipod 5 be available in Australia?
Which sensor works with Omnipod 5?
Who can use the Omnipod 5 System?
How can my patients be kept up to date with the latest information regarding Omnipod 5?
References:
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Median time in hyperglycemic range (>10.0 mmol/L) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs. 29.7%, P<0.0001, respectively. Median time in hypoglycemic range (<3.9 mmol/L) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.0% vs. 1.1%, P<0.0001; 1.4% vs. 1.5%, P 0.8153, respectively. Study funded by Insulet. Study materials provided by Insulet
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time in hyperglycemic range (>10.0 mmol/L) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs 29.5%, P<0.0001, respectively. Median time in hypoglycemic range (<3.9 mmol/L) as measured by CGM in children ST vs. 3-mo Omnipod 5: 2.19% vs. 1.94%, P = 0.0204. Study funded by Insulet. Study materials provided by Insulet.
*The Pod has an IP28 rating for up to 7.6 metres for up to 60 minutes. The Omnipod 5 Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
** When used in automatic mode with a compatible CGM, the Omnipod 5 System makes adjustments to insulin delivery every 5 minutes based on the user's current CGM value, glucose values predicted 60 minutes in the future, glucose trend, and past insulin delivery to bring glucose to a user defined target.
† Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
Important Safety Information: The Omnipod® 5 Automated Insulin Delivery System is indicated for use by individuals with Type 1 diabetes mellitus in persons 2 years and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription and/or ongoing supervision of a qualified healthcare provider. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®/ Novorapid®, Humalog®, Trurapi®/Insulin aspart Sanofi®, Kirsty®, and Admelog®/Insulin lispro Sanofi®.
Refer to the Omnipod 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.
Warning: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycaemia or hyperglycaemia.

