How the Omnipod DASH® System may help improve Quality of Life1

Omnipod DASH Simplifies Diabetes Management1
When treating people living with T1D, one of the considerations is their ability to be healthy, comfortable, and enjoy life events. Fortunately, the Omnipod DASH® System, which consists of a lightweight, smartphone-like Personal Diabetes Manager (PDM) and wearable Pod2, has been shown to increase Quality of Life1.
The information shown below features studies conducted with either Omnipod System or Omnipod DASH. The two devices are very similar in functionality by providing continuous subcutaneous insulin infusion however the Omnipod System was never made available in Australia.
Efforts to prevent complications of diabetes often overlook its effect on Quality of Life (QoL)3
When treating T1D, improvements in treatment satisfaction are often viewed as improvement in overall QoL. This fails to take into account the limited scope of the satisfaction measure. To measure quality of life, it is necessary to not only take into account treatment satisfaction but also the impact that diabetes and its treatment can have on other factors that could affect Quality of Life3.
After three months on Omnipod DASH, 85% of Australians reported an improved quality of life1.
Study descriptions

Study description
Treatment Satisfaction
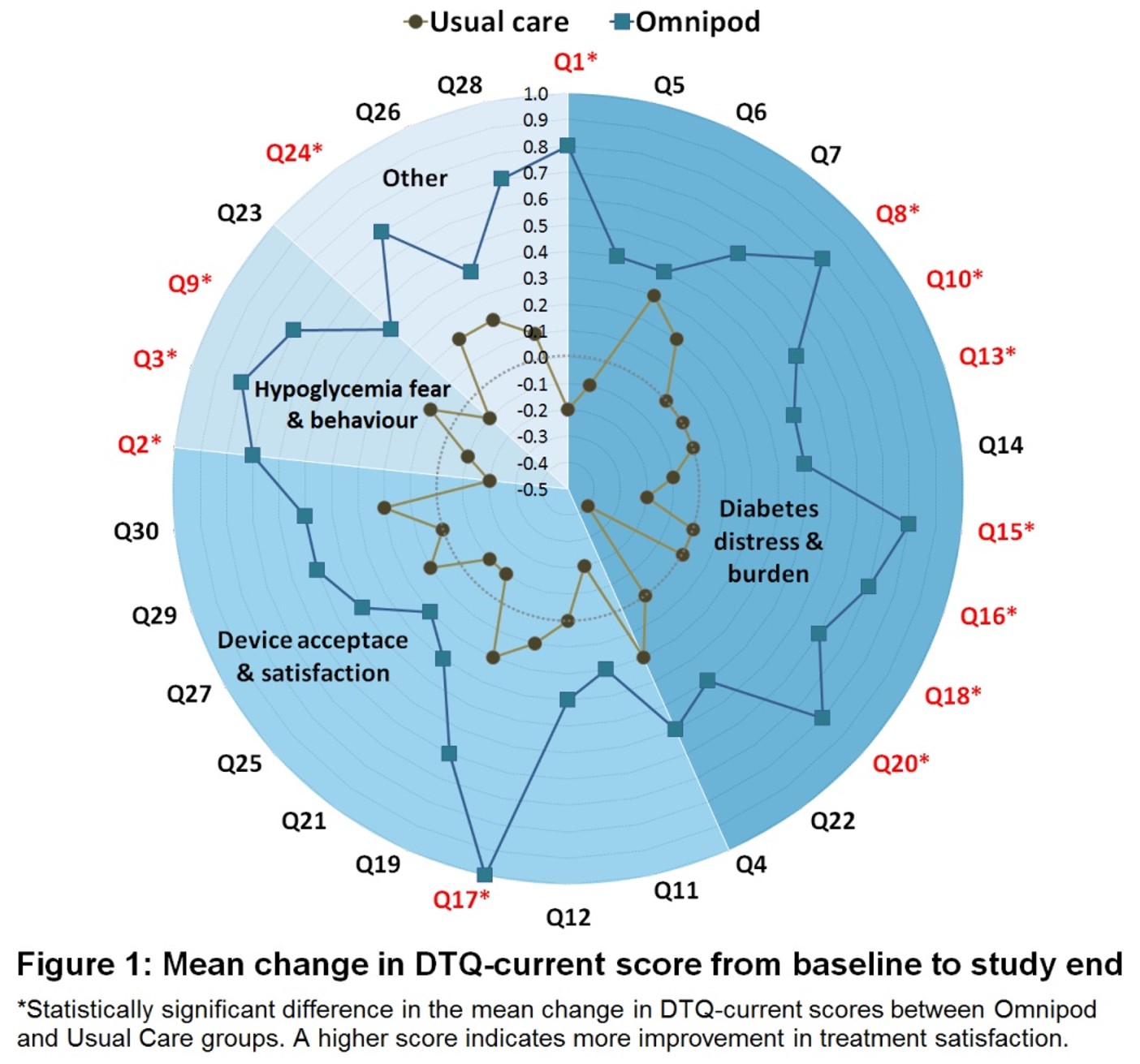
Australian Omnipod DASH® users reported improvements in multiple aspects of Diabetes Technology Questionnaire (DTQ)-current, particularly in the areas of diabetes distress/burden and hypoglycaemia fear/behaviour4.
95% of Australian adults interviewed with T1D using Omnipod DASH® would recommend it to others for T1D management5
Study description
Could your patient's insulin delivery experience be improved with the Omnipod DASH® system?
Omnipod DASH Users
Experience a reduction in HbA1c and diabetes distress compared to usual care (MDI/CSII)4.
Study description
85% Of Users
who switched to Omnipod DASH reported an improved quality of life1.
Study description
An Australian
Randomised controlled trial of Omnipod DASH ®, tubeless pump therapy was associated with greater treatment satisfaction compared with MDI or CSII for adults living with type 1 diabetes4.
Study description
See other data
View our other clinical studies pages, which provide insight into both Australian data and the international experience of the Omnipod DASH System.
Arrange a meeting with an Omnipod® representative
Do you have questions about the Omnipod DASH® System? Our team can provide you with the information you need and help your patients to get started with Omnipod DASH® System.
Be the first to know
Stay up to date and be in the know when it comes to all things Insulet. By signing up for our mailing list, you will be in the know when it comes to training and events, Omnipod® published data and first-hand experiences from PoddersTM.
Important Safety Information: The Omnipod DASH®Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. The Omnipod DASH® System has been tested and found to be safe for use with the following U-100 insulin: Novolog®, Humalog®, Apidra®Fiasp®or Admelog®. Refer to the Omnipod DASH® Insulin Management System User Guide for complete safety information including indications, contraindications, warnings, cautions, and instructions.
References: 1. Nash B, et al. Real-world outcomes following initiation of the Omnipod DASH tubeless insulin pump for people with type 1 diabetes (T1D) in Australia Poster ATTD 2024 E poster EV338 / #502. 2. Omnipod DASH® Insulin Management System [User Guide]. 2020. 3. Bradley, C. et al. Diabetes Metab Res Rev. 18: S64-S69. 4. Kong YW, et al. Treatment satisfaction with Omnipod DASH in adults with type 1 diabetes: A non-blinded 1:1 randomized controlled trial. J Clin Endocrinol Metab. DOI: https://doi.org/10.1210/clinem/dgae088. Published online 19 Feb 2024. A randomised controlled trial of 65 Adults with type 1 diabetes in Australia. 3 months randomisation to either Omnipod DASH® or usual care (MDI:60%/CSII:40%). Primary outcome: treatment satisfaction assessed by change in current Diabetes Technology Questionnaire (DTQ) score. HbA1c, CGM metrics and other questionnaires were also measured. Change in HbA1c (%), Omnipod DASH® -0.4 v usual care -0.1 (P= 0.037). This study received financial and in-kind support from Insulet Corporation. 5. Stocco et al 2023 Diabetes Research & Clinical Practice “You can hide it if you want to, you can let it be seen if you want to”: A qualitative study of the lived experiences of Australian adults with type 1 diabetes using the Omnipod DASH® system https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(24)00036-6/pdf.