How the Omnipod DASH® System may be associated with a reduction in complications of Type 1 diabetes1
When patients living with Type 1 diabetes struggle to reach their recommended glycaemic goals, it may lead to serious consequences2. The Omnipod® System may lead to improved glycaemic control3,4, and a better Quality of Life5. The Omnipod® System was also associated with reduced frequency of acute complications of Type 1 diabetes such as hypoglycaemia and Diabetic ketoacidosis (DKA)1.
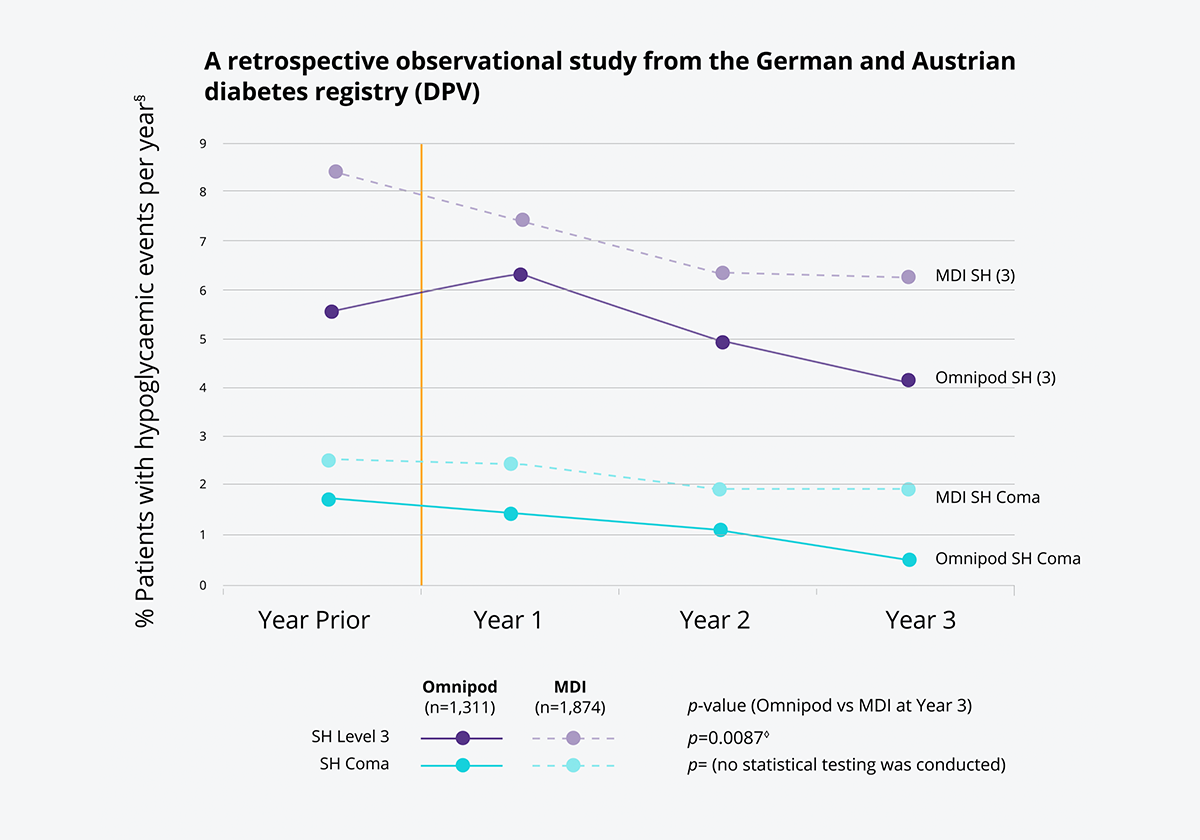
The Omnipod® System was associated with significant reductions in severe hypoglycaemic (SH)* episodes for people with Type 1 diabetes vs multiple daily injections (MDI)1†‡

Retrospective observational study
A retrospective observational study from the German and Austrian Diabetes Registry (DPV) showed that at year 3, the frequency of severe hypoglycaemia requiring assistance was significantly lower in Omnipod® System users compared to MDI users (annual rate of severe hypoglycaemia was 4.1% for Omnipod® System users vs 6.3% for MDI users).
The frequency of severe hypoglycaemic coma was low (1.7% with prior treatment), but continued to decrease each year over the 3 years of tubeless pump use (1.4% at year 1, to 1.1% at year 2 to 0.5% at year 3).
Study design
How the Omnipod® System was associated with a reduction in instances of Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) is the most common acute cause of morbidity and mortality in youth with Type 1 diabetes.9


The Omnipod® System was associated with significant reductions in DKA for people with Type 1 diabetes vs MDI1*†
A retrospective observational study from the German and Austrian Diabetes Registry (DPV) showed that at year 3, DKA frequency was significantly lower in Omnipod® System users compared to MDI users (2.2% of Omnipod® System patients vs 3.3% of MDI users experienced at least one DKA event per year).1
Study design
See other data
The Omnipod® System has also been shown to improve Quality of Life5 as well as glycaemic control.3,4
Contact our Medical Affairs team
At Insulet, our aim is to keep on improving the lives of those living with T1D. With this in mind we aim to generate real-world data to provide further evidence on the efficacy of Pod Therapy. If you want to know more about our current trials and development pipeline do not hesitate to contact our Medical Affairs team at [email protected]
Arrange a meeting with an Omnipod® representative
Do you have questions about the Omnipod DASH® System? Our team can provide you with the information you need and help your patients to get started with Omnipod DASH® System.
Be the first to know
Stay up to date and be in the know when it comes to all things Insulet. By signing up for our mailing list, you will be in the know when it comes to training and events, Omnipod® published data and first-hand experiences from Podders®.
References: 1. Biester T, et al. Diabetes Technol Ther. 2021. 2. Skyler JS. Endocrinol Metab Clin North Am. 1996;25(2):243-25. 3. Mehta AN, et al. Clinical Diabetes. 2020 4. Brown R.E. et al. Diabet Med. 2020 Oct 11:e14420. Advance online publication. 5. Polonsky WH, et al. Diabetes Technol Ther. 2016;18(10):664-670. 6. Foster NC, et al. Diabetes Technol Ther. 2019;21(2):66-72. 7. Renard E, et al. Diabetologia. 2019;62(suppl 1):s8-s9. 8. Kalra, Sanjay et al. Indian journal of endocrinology and metabolism vol. 17,5 (2013): 819-34. 9Ahrén B. Vasc Health Risk Manag. 2013;9:155-163. 9. Aye et al. Diabetes Care. 2019 Mar; 42(3): 443-449.