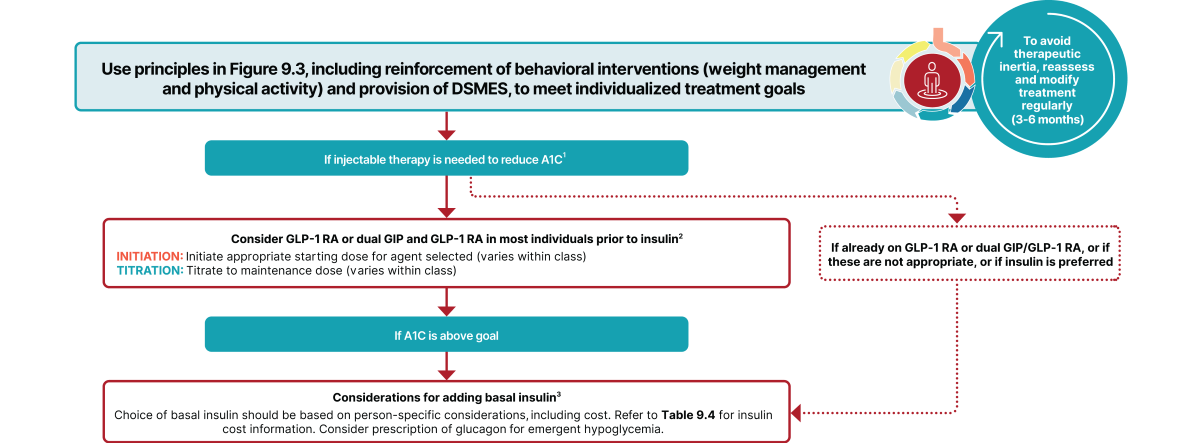
Excerpt from Figure 9.4 from the ADA’s 2025 Standards of Care in Diabetes2:
Also, ADA indicates that insulin has a “high to very high glucose lowering efficacy” for type 2 diabetes, as seen in the comparison chart of medications1
In cases where there is no evidence of insulin deficiency, the ADA prefers a GLP-1 RA, including a dual GIP and GLP-1 RA, as the first injectable therapy to try.1
Changes to Insulin Pump Recommendation in T2D
For the first time, the ADA recommends that “Insulin pump therapy, preferably with CGM, should be offered for diabetes management to youth and adults on MDI with type 2 diabetes who can use the device safely”2 or have a caregiver use the device safely. This is a stronger recommendation than in the previous year when the ADA suggested that insulin pump therapy can be offered.
Insulin pumps are not only a more convenient delivery method than syringes and pens but can also increase patient compliance and satisfaction.2 Continuous glucose monitoring (CGM) adds the benefits of measuring glucose every few minutes and sending glucose level information wirelessly to a companion device or smartphone. Further, within an AID system, the pump uses that real time glucose information , to determine how much insulin to deliver. The amount of insulin delivered will adjust as the glucose values change. Additional mealtime or bolus insulin can be adjusted by the user as needed.
The ADA recognizes that “the use of insulin pumps and AID systems in type 2 diabetes is still limited, and at this time only one system is FDA approved for use in type 2 diabetes”2 which is Omnipod® 5.
About Omnipod 5
Omnipod 5 is the first AID system indicated for people with type 2 diabetes in the U.S. In a clinical study, Omnipod 5 lowered A1C regardless of GLP-1 use and increased participants’ time in range by nearly 5 hours a day.3 For more details of the Omnipod 5 SECURE-T2D clinical study visit Omnipod’s Type 2 Diabetes page.3
Key Takeaways
- The updated ADA guidelines bring important changes from previous years that are poised to significantly change the perception of insulin use and initiation in Type 2 diabetes treatment.
- The ADA recognizes insulin deficiency in patients with T2D as a scenario where insulin can be considered first, or in combination with other therapies, recognizing that these patients may benefit from insulin earlier in the progression of this disease.
- The gap in insulin pump adoption in T2D vs T1D is positioned to shrink. AID technology is already preferred by the ADA as the standard of care for delivering insulin to youth and adults with type 1 diabetes.2
- Technology has the potential to simplify therapy and improve user satisfaction2 and the ADA’s updated guidelines can help provider/T2D patient teams feel confident in introducing an insulin pump and AID system technology in 2025.
- Real world evidence has shown that insulin pumps used to treat T2D are capable of reducing A1C levels and daily insulin dosages2,4
More Resources
1. American Diabetes Association Professional Practice Committee; 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 1 January 2025; 48 (Supplement_1): S181–S206. https://doi.org/10.2337/dc25-S009
2. American Diabetes Association Professional Practice Committee; 7. Diabetes Technology: Standards of Care in Diabetes—2025. Diabetes Care 1 January 2025; 48 (Supplement_1): S146–S166. https://doi.org/10.2337/dc25-S007
3. Pasquel FJ, et al. Presented at: ADA; June 21-24, 2024; Orlando, FL. Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time in range (70-180 mg/dL): standard therapy vs. 13-week Omnipod 5: 45% vs. 66%, P<0.001. In a subgroup analysis of 164 participants who used GLP-1 RA mean HbA1c: baseline vs. 13-week Omnipod 5: 8.1% vs. 7.3%; compared to 132 participants who did not use GLP-1 RA mean HbA1c: baseline vs. 13-week Omnipod 5: 8.4% vs. 7.5% Interaction p-value p= 0.5
4. Carlson AL, Huyett LM, Jantz J, Chang A, Vienneau T, Ly TT. Improved glycemic control in 3,592 adults with type 2 diabetes mellitus initiating a tubeless insulin management system. Diabetes Res Clin Pract 2021;174:108735