Omnipod® 5


Celebrate Simplicity
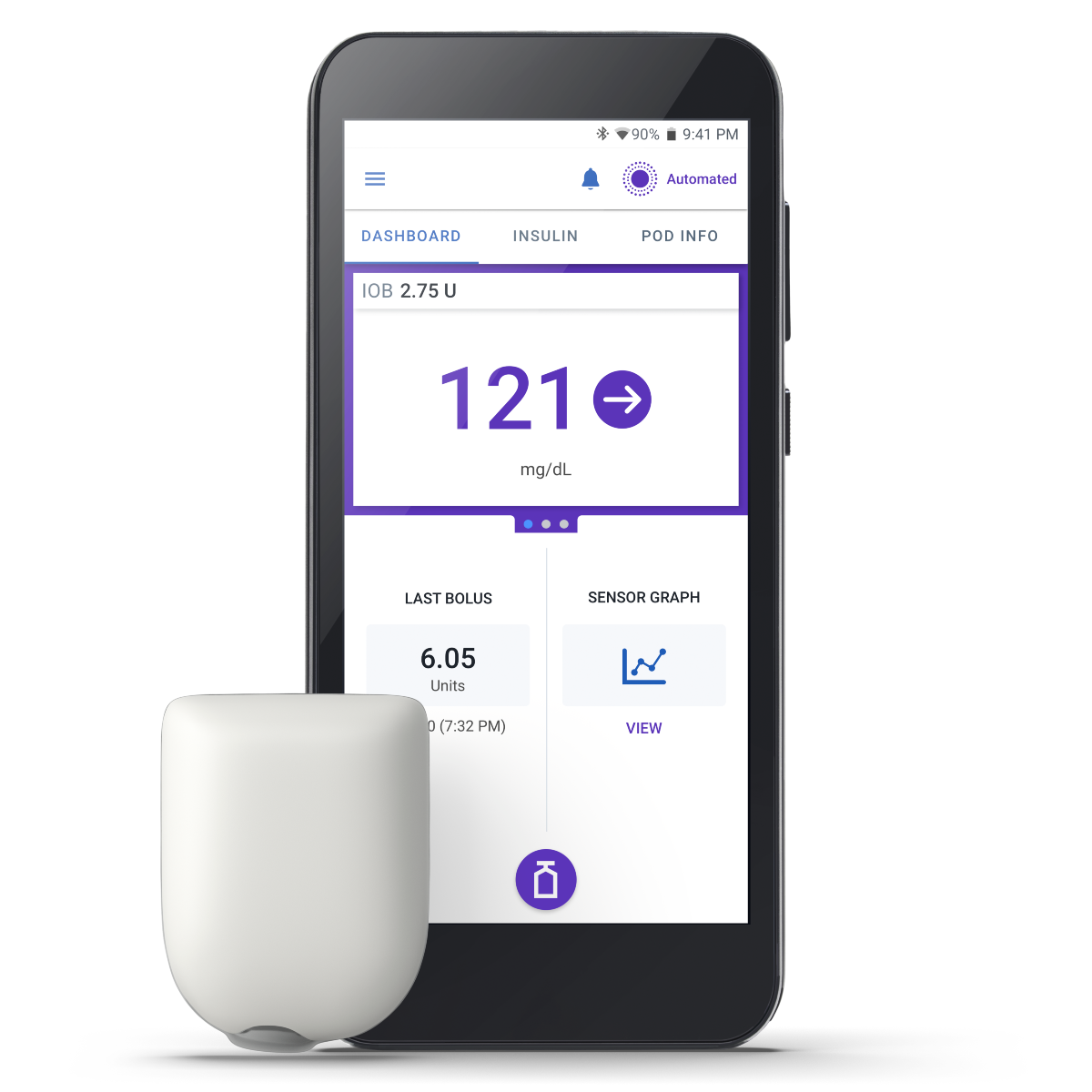
Omnipod 5 is the first and only tubeless automated insulin delivery system in the US market. Give your patients simplified insulin management and accessibility in addition to demonstrated clinical results1,2,3,4
- Indicated for type 1 diabetes for ages 2+, and type 2 diabetes for ages 18+
- Freedom from tubes ideal for multiple daily injection (MDI) users, adults, and pediatrics alike
- Waterproof, on-body experience5
- Now integrated with the Dexcom G6, the Dexcom G7, and the FreeStyle Libre 2 Plus Sensor
- The Omnipod 5 App for iPhone is currently only integrated with Dexcom G6 and compatible with iOS 17 and iOS 18, depending on your iPhone model.*
- Simple to access through the pharmacy without a four year DME lock-in period
Celebrate Patient Success
- Patients previously on MDI switching to Omnipod 5, have seen success achieving a time in range of 70.8% and time below range of 0.96% at an average target of 110mg/dL.6
- AID all day long— there is no reason to disconnect with Omnipod 5 resulting in users experiencing 93.7% median time spent in Automated Mode in a real-world setting.7
- In clinical studies, Omnipod 5 users improved their HbA1c.2,3,4
Hear from colleagues who not only prescribe Omnipod 5, but use it themselves.
How Does It Work?
SmartAdjust™ so you don’t have to!6 The Omnipod® 5 System provides personalized and adaptive insulin delivery using the SmartAdjust algorithm that is built into the Pod.
- Adjusts basal insulin automatically. No need to fine-tune basal settings with SmartAdjust™ technology. With every pod change, it adapts to your patient’s dynamic insulin needs in daily life and can also cover long-term developments like changes in weight, growth, and aging.8
- Corrects high glucose values with microbolusing. Every 5 minutes SmartAdjust™ technology delivers a newly calculated microbolus, based on current and predicted glucose, to manage hyperglycemia. Corrections can be as high as four times the adaptive basal rate.
- Protects by decreasing or pausing insulin delivery. SmartAdjust™ technology helps to protect against hypoglycemia by decreasing or pausing insulin if hypoglycemia is experienced or predicted.2,3,4
For more in-depth information, watch our Algorithm Overview Video
Want to get a hands-on understanding of our intuitive Omnipod 5 AID system?
Try the free simulator app on your smartphone.
Ready to Prescribe?
We offer not only a streamlined prescribing process through the pharmacy, but provider resources to help you support your patients.
*For a full list of compatible phones, visit omnipod.com/compatibility.
1. Cleared for people living with type 1, ages 2+ and type 2, ages 18+. Only available for users with valid prescription and coverage through their pharmacy benefit. Exact coverage depends on patient’s insurance plan. Upgrades subject to user’s insurance coverage.
2. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs [adults/adolescents (n= 128; aged 14-70 yrs) children (n=112; aged 6-13.9 yrs)]. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop phase. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. HbA1c in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 7.16% vs 6.78%, P<0.0001; 7.67% vs 6.99%, P<0.0001, respectively. Mean time 70-180mg/dL as measured by CGM in adults/adolescents and children, ST vs. Omnipod 5: 64.7% vs. 73.9%, P<0.0001; 52.5% vs. 68.0%, P<0.0001, respectively. Mean time >180 mg/dL in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 32.4% vs. 24.7%; 45.3% vs. 30.2%, P<0.0001, respectively. Median time <70 mg/dL in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 2.0% vs. 1.1%, P<0.0001; 1.4% vs. 1.5%, P=0.8153, respectively. Results measured by CGM.
3. Sherr JL, et al. Prospective trial in 80 participants with T1D aged 2 - 5.9 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean HbA1c: ST vs. Omnipod 5 use in very young children (2 - 5.9 yrs) 7.4% vs 6.9%, P<0.05. Mean time in range (70-180mg/dL) in very young children (2 - 5.9 yrs) as measured by CGM: ST = 57.2%, 3-mo Omnipod 5 = 68.1%, P<0.05. Mean time >180 mg/dL in very young children (2 - 5.9yrs) as measured by CGM: ST = 39.4%, 3-mo Omnipod 5 = 29.5%, P<0.0001. Mean time <70 mg/dL in very young children (2-5.9 yrs) as measured by CGM: ST = 3.41%, 3-mo Omnipod 5 = 2.13%, P=0.0185. Results measured by CGM.
4. Pasquel FJ, et al. Presented at: ADA; June 21-24, 2024; Orlando, FL. Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time in range (70-180 mg/dL): ST vs. 13-week Omnipod 5: 45% vs. 66%, P<0.001. Mean HbA1c: ST vs. 13-week Omnipod 5: 8.2% vs. 7.4%, P<0.001. Mean time >180 mg/dL as measured by CGM: ST = 54%, 3-mo Omnipod 5 = 34%, P<0.001. Mean time <70 mg/dL as measured by CGM: ST = 0.2%, 3-mo Omnipod 5 = 0.2%
5. The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Controller is not waterproof.
6. Forlenza G, et al. Diabetes Technol Ther (2024). 6,525 Omnipod 5 users with type 1 diabetes at the Target Glucose of 110 mg/dL who utilized MDI as prior therapy had a time in range of 70.8% and time below range of 0.96%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available."
7. Forlenza G, et al. Real-world evidence of Omnipod 5 Automated Insulin Delivery System use in 69,902 people with type 1 diabetes resulted in 93.7% time in Automated Mode. Diabetes Technol Ther. 2024.
8. In Automated Mode, SmartAdjust technology uses total daily insulin (TDI) to set a new Adaptive Basal Rate. Omnipod 5 User Manual. P. 291