Omnipod® 5
“Unlock the Future of Type 2 Diabetes Care”
featuring Dr. Viral Shah
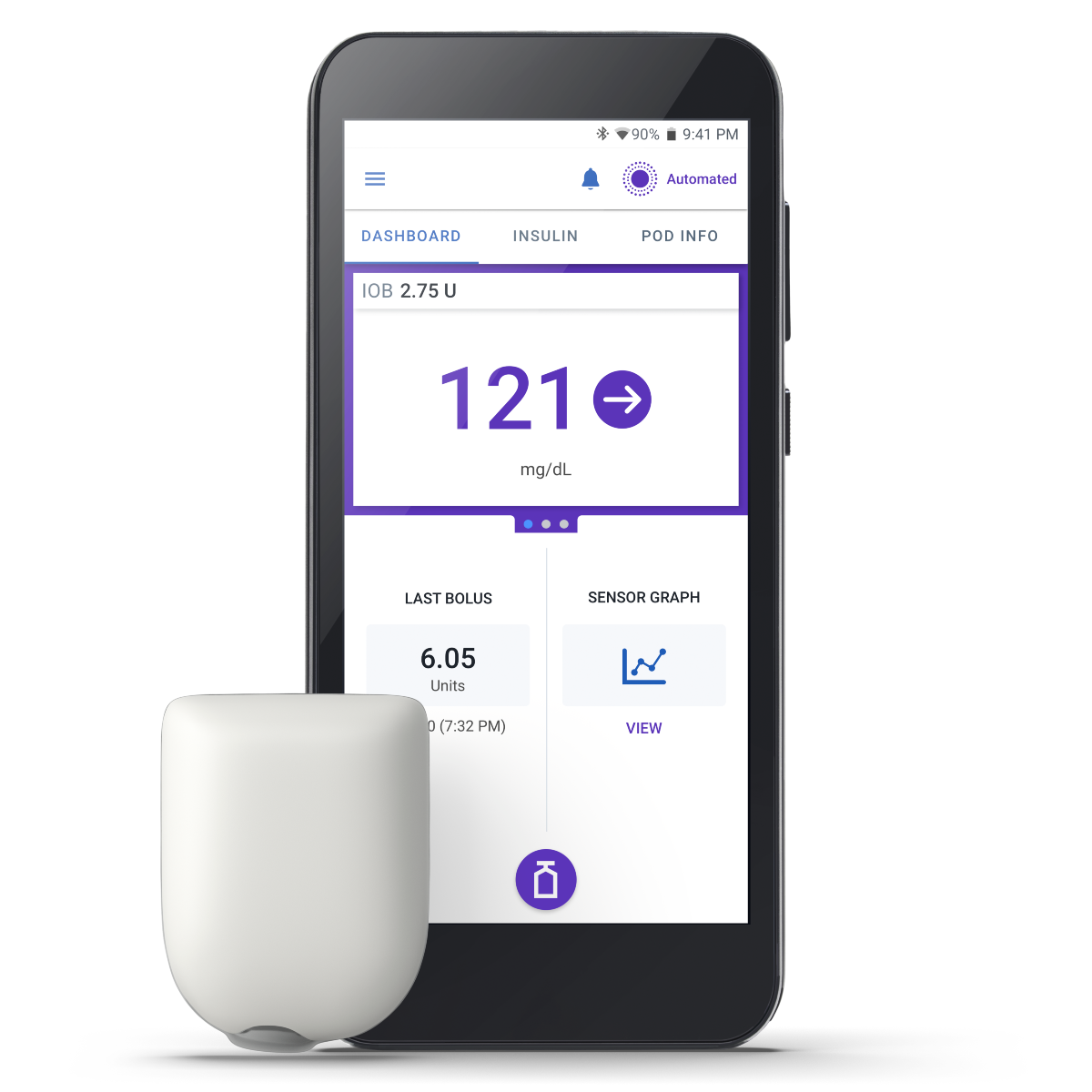

Omnipod 5 is the first and only automated insulin delivery (AID) system to be FDA-cleared for adults living with type 2 diabetes in the US!
Omnipod 5 offers simplicity, clinical results, and broad access so that you can offer your patients the technology that is right for them.
Omnipod 5 Demonstrated Improved Results for People Living with Type 2 Diabetes
The Omnipod 5 SECURE-T2D clinical study is the first and most racially diverse pivotal study of AID in T2D published to date.1
46%
of study participants were Black, Hispanic, or Latino2
55%
of study participants were using a GLP-12
84%
of study participants not carb-counting before enrollment2
- Lowered A1C regardless of patients’ race, ethnicity, GLP-1 use, and whether they were carb counting or not. In patients with a baseline of A1C ≥9%, the A1C was lowered by 2.1% (-0.8% in full study population)2
- More time in range by nearly 5 hours a day (% of time spent between 70-180 mg/dL)2
- No demonstrated increase in time in hypoglycemia (% time spent <70 mg/dL)2
- 29% reduction in insulin need2
One case of severe hypoglycemia was reported by an adult with type 2 diabetes during Omnipod 5 System use. The case was not related to Omnipod 5 System malfunction.
Interested in understanding the SECURE-T2D study in greater detail? Watch clinical investigators discuss results.
“Advancing Toward AID for All”
SECURE-T2D study excerpt by Dr. Francisco Pasquel from the Omnipod 5 Product Theater that was recorded at the American Diabetes Association’s 84th Scientific Sessions Meeting in Orlando, FL.
Download the Omnipod 5 brochure, including a study overview.
SECURE-T2D Study Participants Had a High Level of Satisfaction with Omnipod 5
Participants in the Omnipod 5 SECURE-T2D study reported the following:
- Clinically meaningful reduction in diabetes distress2
- Simple bolusing—over 91% said it was easy to use the SmartBolus Calculator3
- 90% would recommend Omnipod 5 to a friend or family member3
- 72% said they barely noticed wearing the discreet, tubeless and waterproof4 system3
- Continued use—78% said that they would like to continue to use Omnipod 5 after the study3

“I am a nurse and know the importance of controlling my diabetes but unfortunately, I would miss my insulin injections … I was on a dangerous path.
With Omnipod 5, I have far fewer ups/downs of high and low sugars, and along with it, the terrible symptoms associated with that. Omnipod has been life changing for me.”5
—Milli, Omnipod 5 User with type 2 diabetes
Mili has an ongoing commercial relationship with Insulet.
Omnipod 5 Has Broad Access Through the Pharmacy
- The only AID system covered under Medicare Part D without a C-peptide test
- Simple pharmacy access means that patients can pick up Pods when they pick up their insulin
- A majority of Omnipod 5 users with type 2 diabetes pay less than $50 a month6
Ready to take the next steps?
Have questions about type 2 indication?
Want to learn more about
Omnipod 5?
Do you have patients who can benefit?
Subscribe to Stay in Touch
Our newsletter for healthcare providers includes the latest Omnipod news and educational resources.
2. Pasquel FJ, et al. SECURE-T2D clinical study. JAMA Network Open (2025). Subgroup analysis of 68 participants with baseline A1C ≥ 9% Mean HbA1c: standard therapy vs. 13-week Omnipod 5: 10.1% vs. 8.1%; (95% CI: -2.3%, -1.9%). Mean time in range (70-180 mg/dL): standard therapy vs. 13-week Omnipod 5: 45% vs. 66%, P<0.001. Mean time <70 mg/dL as measured by CGM: standard therapy = 0.2%, 3-mo Omnipod 5 = 0.2%. Mean total daily dose (TDD): ST = 0.8U/kg/day, 3-mo Omnipod 5 = 0.57U/kg/day, P<0.001. Comparison is relative change.
3. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean T2-DDAS total intensity score: ST = 2.5, 3-mo Omnipod 5 = 2.2, P<0.001. Mean Proportion with T2-DDAS ≥ 2.0, no. (%): ST = 66%; 3-mo Omnipod 5 = 55%, P<0.001. 78% of participants strongly agreed and 12% agreed a little that they would recommend Omnipod 5 to family member or friend. 74% of participants strongly agreed and 16% agreed a little that it is easy to use the SmartBolus Calculator. 65% of participants strongly agreed and 13% agreed a little that they want to continue using the Omnipod 5 System after the trial. 72% of participants said they barely noticed wearing the system.
4. The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Omnipod 5 Controller is not waterproof.
5. Source: IQVIA OPCL. Majority is defined over 70%. Based on paid claims for Omnipod 5 G6 Pods with a type 2 diabetes diagnosis code. Includes commercial and Medicare claims January 2023 through December 2023.
INS-OHS-10-2024-00078 V3.0