A Simplified Way to Prescribe Omnipod® 5
“We now have 90% of covered lives. So, what that means: more than 300 million people in the United States can have access to Omnipod 5. … We do that because Omnipod 5 is accessible through the pharmacy … for our users, it means they’re not locked into a four year period through DME.” —Dr. Trang Ly
Omnipod is available through the pharmacy:
- Your patients can get started today1—more than half of covered Omnipod 5 scripts are filled within 24 hours through the pharmacy2
- The majority of Omnipod 5 customers pay $50 or less per month3
1Only available for users with valid prescription and coverage through their pharmacy benefit. Exact coverage depends on patient’s insurance plan. Upgrades subject to user’s insurance coverage.
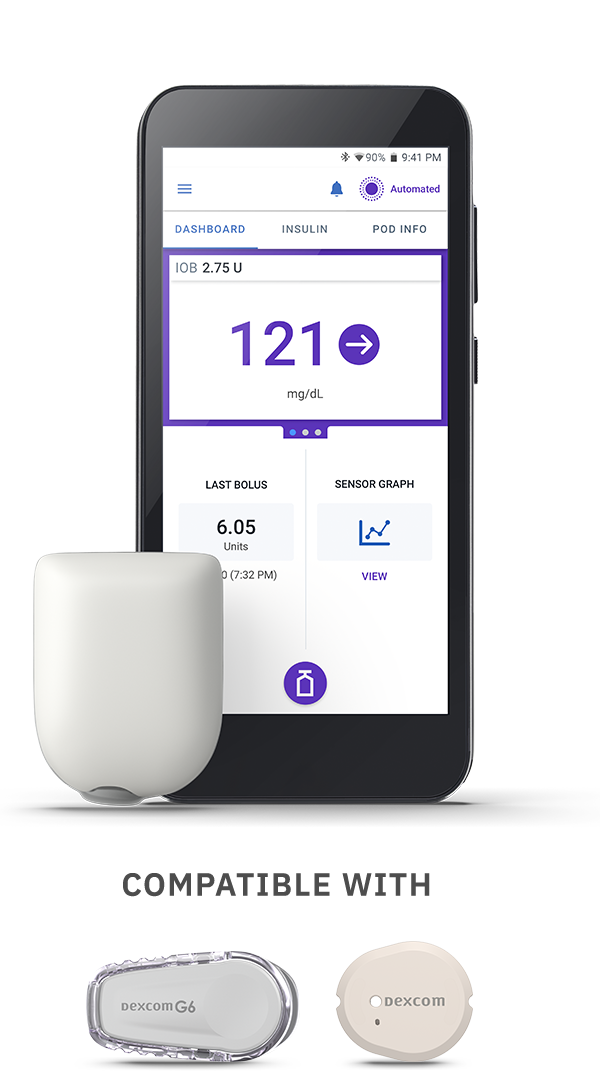

e-Prescribe to your patient’s preferred pharmacy:
Both of the following prescriptions need to be written to either your patients’ preferred pharmacy or to ASPN Pharmacies, LLC (New Jersey location) to get your patient started on the Omnipod 5 Automated Insulin Delivery (AID) system*
| Product Description | Package Contents | Quantity | Refills | Dosing/RX SIG Instructions |
| Omnipod 5 DexG7G6 Intro Kit (Gen 5) OR Omnipod 5 G6 Intro Kit (Gen 5) NDC/NRC: 08508-3000-01 | Controller and 10 Pods | 1 Kit | None | Change Pod every 72 or 48 hrs** Based on total daily insulin usage |
| Omnipod 5 DexG7G6 Pods (Gen 5) OR Omnipod 5 G6 Pods (Gen 5) NDC/NRC: 08508-3000-21 | 5 Pods per box | 2 boxes | 1 Year Monthly refills | Change Pod every 72 or 48 hrs ** Based on total daily insulin usage |
** If patient requires a 48-hour Pod change frequency, quantity should be 3 boxes. Clinical rationale must be provided for 48-hour Pod changes.
Obsolete NDC/NRC Codes to Avoid
As EHR systems don’t have a centralized approach to version control, your clinic may encounter several obsolete Product Names and NDCs/NRCs when prescribing Omnipod. Below are obsolete names and codes to help you avoid prescribing them in error.
| NDC/NRC | Product Name | Contents |
| 08508-3000-50 | Omnipod 5 G7 Intro Kit (Gen 5) | 1 Controller & 10 Pods |
| 08508-3000-53 | Omnipod 5 G7 Pods (Gen 5) | 5 Pods |
| 08508-3000-50 | Omnipod 5 G6-G7 Intro Kit (Gen 5) | 1 Controller & 10 Pods |
| 08508-3000-53 | Omnipod 5 G6-G7 Pods (Gen 5) | 5 Pods |
Are your patients on the fence? They may be eligible for a 30-day Free Trial.
Financial Assistance Programs May Be Available5
Have your patients call customer care at 1-800-591-3455 to learn more.
2. Calculated based on a consumption of ten (10) Pods per month. Majority defined as at least 70% of patient co-pays $50 or less per month . Among All Paid Omnipod 5 G6 Pods Commercial and Medicare Claims from August 2022 through July 2023. Includes benefits and offerings available through Insulet, such as the copay card program. Actual co-pay amount depends on patient’s health plan and coverage, they may fluctuate and be higher or lower than the advertised amount on a monthly basis. Source: IQVIA OPC Library. Calculated based on a consumption of ten (10) Pods per month. Among All Paid Omnipod 5 G6 Pods Commercial and Medicare Claims from August 2022 through July 2023. Includes benefits and offerings available through Insulet, such as the copay card program. Actual co-pay amount depends on patient’s health plan and coverage, they may fluctuate and be higher or lower than the advertised amount on a monthly basis. Source: IQVIA OPC Library
3. Calculated based on the proportion of script fills within 24-hour look forward period out of all script fills within a 90-day look forward period among all new to brand claims for the Omnipod 5 G6 Intro Kit from October 2022 to March 2023. Excludes claim reversals. Source: IQVIA Payer Control Library
4. Omnipod® 5 30-Day Free Trial
Terms and Conditions
Omnipod® 5 Intro Kit 30-Day Trial
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod 5 Intro Kit 30-day trial program (the “Program”) is open to patients who have a valid Omnipod 5 prescription as well as a compatible CGM prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. In order to be eligible, the patient’s eligible insurance plan must include coverage for Omnipod 5 Pods. The Program is open to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod 5, Omnipod DASH® or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod 5 or compatible CGM prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs. It is not valid for cash-paying participants or where prohibited by law. A participant is considered cash-paying where the participant has no insurance coverage for Omnipod 5 or where the participant has commercial or private insurance but Insulet determines in its sole discretion the participant is effectively uninsured because such coverage does not provide a material level of financial assistance for the cost of an Omnipod 5 prescription. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Participants receiving their products through the Durable Medical Equipment or Pharmacy Durable Medical Equipment channel are not eligible to participate in the copay card program. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a limited supply of Omnipod 5 products at no cost for them. Eligible participants have two (2) options, based on the following:
• A participant shall sign the Omnipod® 5 Intro Kit 30-Day Trial Acknowledgement through the appropriate platform provided by Insulet.
• Once Insulet has received the request, the request shall be escalated to Insulet’s pharmacy partner, where a request for a prescription shall be sent to the participant’s healthcare professional. If a valid prescription is received, both for the Omnipod 5 Intro Kit and the Omnipod 5 Pods, the participant’s benefits will be checked by Insulet or its partners.
• IF the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod 5 Intro Kit, in accordance with Section 3, below.
• IF the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod 5 Intro Kit, in accordance with Section 4, below.
• For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
• Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
• In order to use the Omnipod 5 System in Automated Mode, the User must also procure a compatible CGM. This program does not include supply of a compatible CGM.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod 5 Copay Card, participant shall receive electronically one (1) Omnipod 5 Copay Card, valid for a single use, in the amount required for the participant to procure one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
- One (1) Controller charging cable
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod 5 Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
- One (1) Controller charging cable
The Omnipod 5 Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.
5. Omnipod® Copay Card
Financial Assistance Program Terms
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod® Financial Assistance Program (the “Program”) is open to patients who have a valid Omnipod DASH® or Omnipod® 5 prescription who demonstrate a financial need for assistance based on criteria established by Insulet, and who fill their prescription through the Pharmacy channel.
This offer is not valid for participants whose prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state program. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Participants receiving their products through the Durable Medical Equipment or Pharmacy Durable Medical Equipment channel are not eligible to participate in the copay card program. Participants on certain commercial insurance plans may not be eligible. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With the Program, an approved participant who meets eligibility criteria may receive a copay card to reduce their monthly out-of-pocket expenses when filling their Omnipod® prescription. The program is described as follows:
• A program benefit that covers the participant’s eligible out-of-pocket prescription costs for Omnipod DASH® and Omnipod® 5 Pods (copay, deductible, or co-insurance) on behalf of the participant, in accordance with criteria determined by Insulet.
• In order to participate in the Program, a person shall complete Insulet’s Financial Assistance Program Application Form, as provided by Insulet and as may be updated from time to time.
• The form shall be filled out with true and correct information by the applicant and provided to Insulet.
• In addition, the applicant shall provide evidence of income, as directed by Insulet.
• Insulet shall evaluate the application in accordance with its policies and make a determination as to the eligibility of the applicant.
• If the application is accepted by Insulet, Insulet shall communicate to the participant the level of assistance that they will receive as part of the Program.
• The assistance shall be provided through a copay card delivered electronically by Insulet to Participant.
• The copay card shall be valid for one (1) year and covers a thirty (30) days’ fill of Pods, every month.
• Participants are solely responsible for updating Insulet with changes to their prescription, financial situation or health insurance, including but not limited to, initiation of insurance provided by the government, in addition to any change in coverage terms or other offers such as accumulator adjustment benefit design or copay maximization programs. Participants shall further inform Insulet of any change or lapse in coverage for their Omnipod ® prescription.
• Participants are responsible to provide Insulet with accurate information on their copay.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Limitations
The Program may not be combined with any other offer, rebate or coupon. If at any point a participant begins receiving coverage under any state or government program, the participant will no longer be able to use this card and they must contact Insulet Customer care at 1-800-591-3455 to stop their participation. Participant shall also update Insulet if their financial situation changes in a way that would make them non-eligible to participate in the Financial Assistance Program. Participating in this Program means that you are ensuring you comply with any required disclosure regarding your participation in the Program. Other restrictions may apply. Health plans, specialty pharmacies and Pharmacy Benefits Managers not specifically authorized by Insulet are prohibited from enrolling participants in the Program. The copay card shall last for a maximum of twelve (12) months per participant.
This Program is not health insurance. Insulet reserves the right to rescind, revoke or amend this offer, as well as any eligibility criterion without further notice.
INS-OHS-04-2022-00006 v7.0