What is Omnipod®?
Omnipod® gives you more with less
Wearable: Place the Pod almost anywhere you’d normally inject insulin
Waterproof*: Take your insulin anywhere life takes you, even while swimming
Tangle-proof: Forget the tubes of traditional insulin pumps
Each wearable Pod gives you up to 3 days (72 hours) of continuous insulin delivery, avoiding the inconvenience and interruptions of up to 14 injections† per Pod.
You’re in control with personalized insulin doses, delivered in just a few finger taps via the Pod’s handheld companion, while the built-in Bolus Calculator takes the headache out of mealtime math.
Simplify diabetes management with Omnipod!
Discover the freedom that Omnipod can offer by registering for a free Pod Therapy 101 webinar.
Pod Therapy: the building blocks of Omnipod
Omnipod offers waterproof*, discreet insulin management through Pod Therapy, an alternative to traditional insulin pumps and multiple daily injections (MDI). Pod Therapy consists of two primary parts: the tubeless Pod and the handheld Personal Diabetes Manager (PDM) or Controller. No multiple daily injections (MDI), tubes, or tangles to hold you back.
89% of new Omnipod customers agree that insulin delivery is easier with the Omnipod DASH Insulin Management System‡
Unlock the Power of Pod Therapy
Meet our tubeless, wearable Omnipod lineup
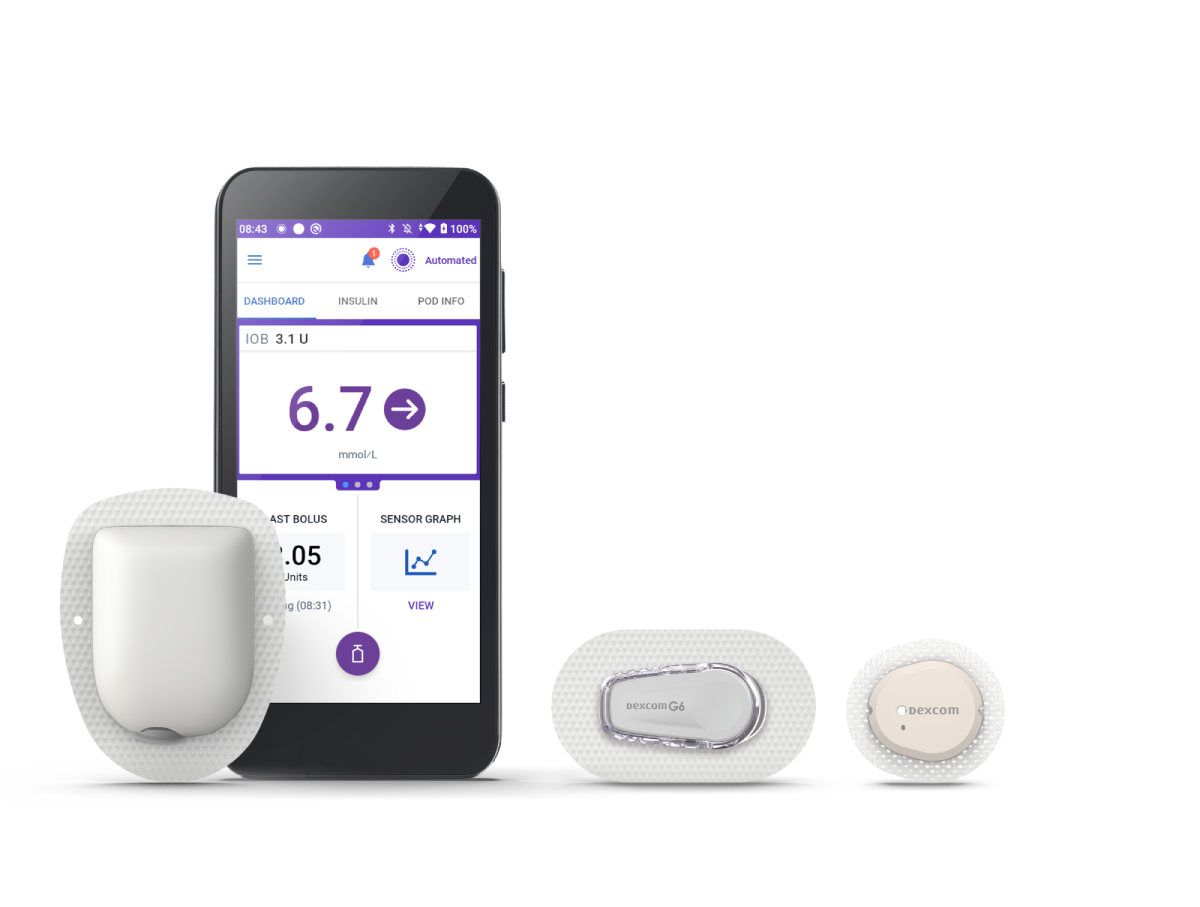

Omnipod® 5
Omnipod 5 is the first and only tubeless automated insulin delivery system that works with Dexcom G6 and G7 Sensors.§
Compatible with Dexcom G6 and G7 Sensors, Omnipod 5 makes automated‡ insulin adjustments every five minutes based on sensor readings, to help keep you in range while lowering HbA1c.1,2
And there’s so much more to Omnipod 5 that can help you to level up your life!
- SmartAdjust™ technology automatically increases, decreases, or pauses insulin every 5 minutes, based on your customized target—helping to protect against highs and lows, day and night. Share the load with the system that never sleeps.1,2
- Activity Feature, when enabled, reduces insulin delivery when glucose typically goes low, for example when exercising, so you can sweat without stress.
- SmartBolus Calculator, the only built-in bolus calculator that automatically incorporates your sensor value and trend, so you don’t have to.
Say hello to Omnipod DASH
Simplify Life with Omnipod DASH. Place the Pod on your body almost anywhere you would inject insulin for up to 3 days (72 hours) of continuous insulin delivery.
The Personal Diabetes Manager (PDM) communicates wirelessly with the Pod, so you can adjust your insulin doses discreetly and easily wherever you are, without the need for tubing.
Say yes to life with Omnipod DASH and discover life, uninterrupted!


"Getting involved in the diabetes universe was a little scary at first, but when you do put yourself out there it’s quite powerful and motivating. Knowing that you are not alone is a powerful feeling, especially if it is with something so life changing as diabetes."
Aubree
Sponsored Podder® since 2022
Like what you see?
Now that you’ve learned a little about Omnipod, you might be ready to take the next step. To get more information on whether Omnipod is right for you, connect with an Omnipod Specialist for more information. Or, if you’re ready to take the Pod for a spin, you can order a free Pod Experience Kit, that includes a needle-free, non-functioning, tubeless Pod that you can wear to get a feel for tubeless freedom.
*The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. Please consult sensor manufacturer user guide for sensor waterproof rating. The Omnipod 5 Controller and the Omnipod DASH PDM are not waterproof.
†14 injections/3 days based on people with T1D on MDI taking ≥ 3 bolus and 1-2 basal injections/day multiplied by 3 days (~4.5/day). Chiang JL, et al. Diabetes Care. 2014;37(7):2034-2054.~
‡Insulet data on file. Data collected from a customer satisfaction survey of 220 participants using the Omnipod DASH Insulin Management System. Data presented by Orchard Hill Market Research on January 5, 2020.
§Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription. Sensors must be used with compatible apps on a supported smartphone.
1. Brown S, et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, p<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, p<0.0001; 2.51% vs. 1.78, p=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, p<0.0001; 2.13% vs. 1.98%, p=0.2545, respectively.
2. Sherr J, et al. Diabetes Care. 2022;45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, p=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, p=0.0799.