Insulin Pod Therapy, Explained
If you’ve read Insulin Pump Therapy for People with Diabetes, you should now know more about what an insulin pump is and how it can help with your diabetes management. In this page we are going to find out more about Pod Therapy with Omnipod®.
What Is Insulin Pod Therapy?
Pod Therapy offers the same benefits of pump therapy, only with a tubeless insulin Pod. This allows you to experience freedom and continue doing the things you love most. Want to know more? Let’s take a look…
Pod Therapy is only found with Omnipod. It’s insulin pump therapy, simplified.
The Omnipod 5 patch pump is an automated insulin delivery system that eliminates the need for multiple daily injections and tubing.
How does Insulin Pod Therapy Work?
Rather than multiple daily injections, or a traditional tubed insulin pump, Pod Therapy delivers insulin via a discreet, wearable device called a Pod.
You can place the Pod almost anywhere you would inject insulin and wear it for up to three days (72 hours) of continuous insulin delivery.
The insulin delivery is wirelessly§ programmed from a separate device. This is done using the Omnipod 5 App on the provided Controller or a compatible smartphone.*
§ At start-up, the Pod and Omnipod 5 App must be adjacent and touching.
*For a full list of compatible smartphones, visit omnipod.com/compatibility.
Is it easy to apply the Pod?
In a word… yes! Just three simple steps* and you’re set for up to three days (72 hours) of continuous insulin delivery.
Step 1. Fill (Fill the Pod)
The Pod automatically primes itself and performs a series of safety checks to prepare for insulin delivery.
Step 2. Place (Apply the Pod)
Place your Pod almost anywhere you would give yourself an injection.
Step 3. Tap (Press Start)
The cannula inserts automatically, and insulin delivery begins at the touch of a button.
Watch this video to learn more about site preparation, how to put the Pod on and how to activate the Pod.
What about Automated Insulin Delivery (AID)?
Omnipod 5, the latest Omnipod system, is an AID system, which you might know as a hybrid closed loop system (HCL). AID allows for a complete circle of communication between the insulin pump or Pod and a continuous glucose monitor (CGM), responding to current and predicted glucose levels by automatically increasing, decreasing or pausing insulin delivery every 5 minutes depending on whether glucose levels are rising or falling.
Omnipod 5 currently connects with the Dexcom G6, Dexcom G7, and FreeStyle Libre 2 Plus Sensor to enable automatic insulin adjustments, which may help to control blood glucose levels, reduce hypoglycemia, and increase time in range.1-3
Sounds good, right? We’ll explore this more in Automated Insulin Delivery (AID), Explained.
Continuous Glucose Monitor and Pod Therapy
A continuous glucose monitor, or CGM, is a small, wearable device that measures your glucose levels every few minutes, day and night.
The CGM sensor sticks to your body with a wire that sits just under the skin. This measures your interstitial glucose levels, automatically sending them to a reader or smartphone - meaning you can check your blood glucose levels at a glance. This can often dramatically reduce the need for fingerstick testing.*
If you want to learn more about glucose monitors first, head to What is a Continuous Glucose Monitor (CGM)?
Pod Therapy with Omnipod 5
The Pod


The Pod is a small, tubeless, wearable and waterproof† device that you fill with insulin and wear directly on your body.
The Pod includes a small, flexible cannula that inserts automatically with the push of a button on the Controller or compatible smartphone.* You never even have to see the needle.
The Pod communicates wirelessly with the Omnipod 5 App on the Controller, or compatible smartphone* to deliver bolus or mealtime insulin.
The Omnipod 5 App
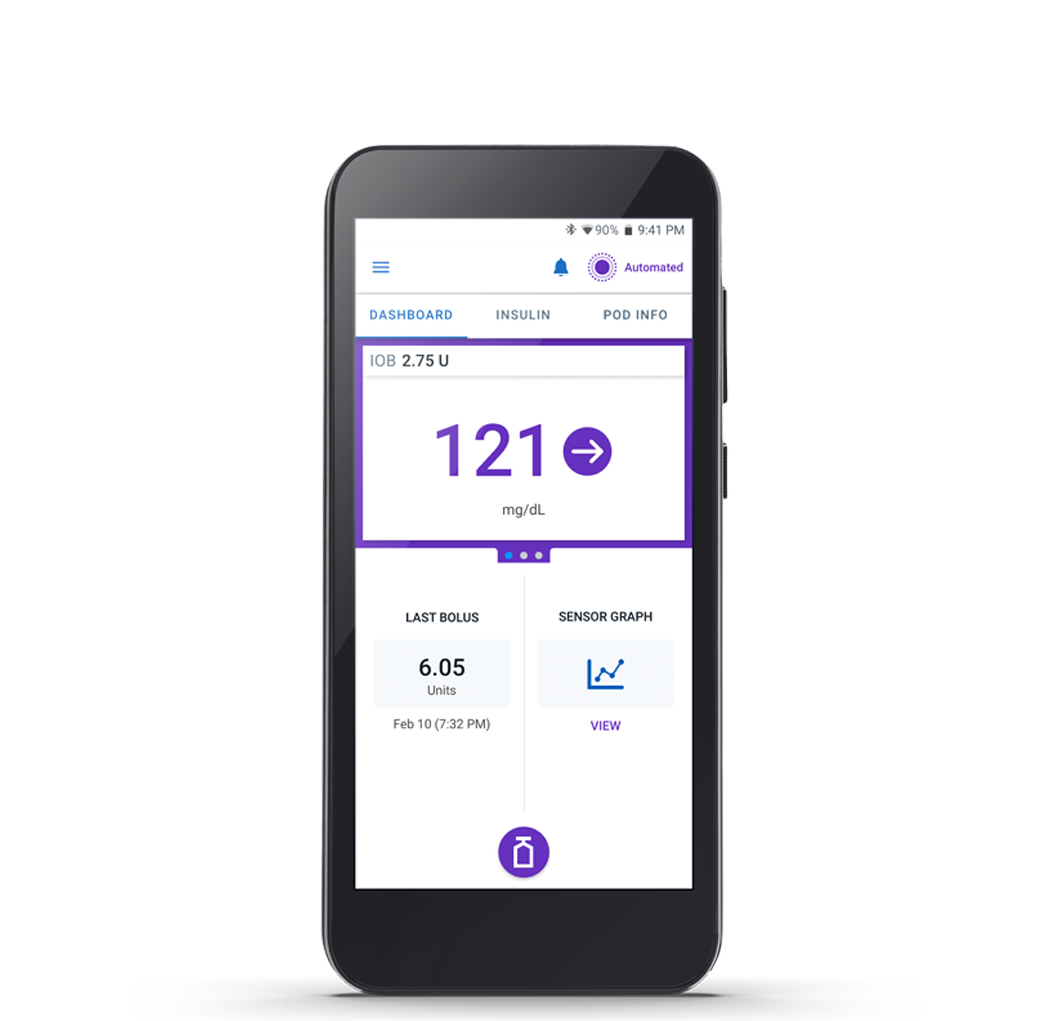

Manage diabetes with freedom and flexibility. Automated insulin delivery is at your fingertips.
- iPhone or Android - Run the Omnipod 5 App on a compatible smartphone* or use the Insulet-provided Controller
- Customizable settings - Personalize your insulin delivery by selecting basal profiles, target glucose levels and bolus settings
- Everyday convenience - Bolus with just a tap using the Omnipod 5 App. Use Custom Foods to simplify mealtime math.
*For a list of compatible smartphone devices visit omnipod.com/compatibility.
Here’s what our Podders have to say about Pod Therapy…
The Omnipod 5 has helped my A1C tremendously. My numbers stay in range more often than not. It has helped my health, my body. The incredible technology is making sure you are living your best life possible.
Garrett V.
Sponsored Podder®
Continue reading:
Related Articles
References and Disclaimers
1. Brown et al. Diabetes Care (2021). Study in 240 people with T1D aged 6 -70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time in Target Glucose range (from CGM) for standard therapy vs Omnipod 5 in adults/adolescents = 64.7% vs. 73.9% and children = 52.5% vs. 68.0%.
2. Sherr JL, et al. Diabetes Care (2022). Study in 80 people with T1D aged 2 –5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time in Target Glucose range (from CGM) for standard therapy vs Omnipod 5 = 57.2% vs. 68.1%.
3. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time <70 mg/dL as measured by CGM: ST = 0.2%, 3-mo Omnipod 5 = 0.2%, P<001. Mean time >180 mg/dL as measured by CGM: ST = 54%, 3-mo Omnipod 5 = 34%, P<0.001. Comparison is relative change
These modules are not a replacement for medical advice or training. Please always speak to a qualified healthcare professional about your options.
The Omnipod® 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and type 2 diabetes mellitus in persons 18 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®, Humalog®, and Admelog®.
Available products subject to current insurance coverage and product indication for use. Insulet can only support onboarding for those customers within the product indication.
Refer to the Omnipod 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions. Warning: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia. WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.

