Omnipod DASH® Insulin Management System
Meet the Omnipod DASH Insulin Management System

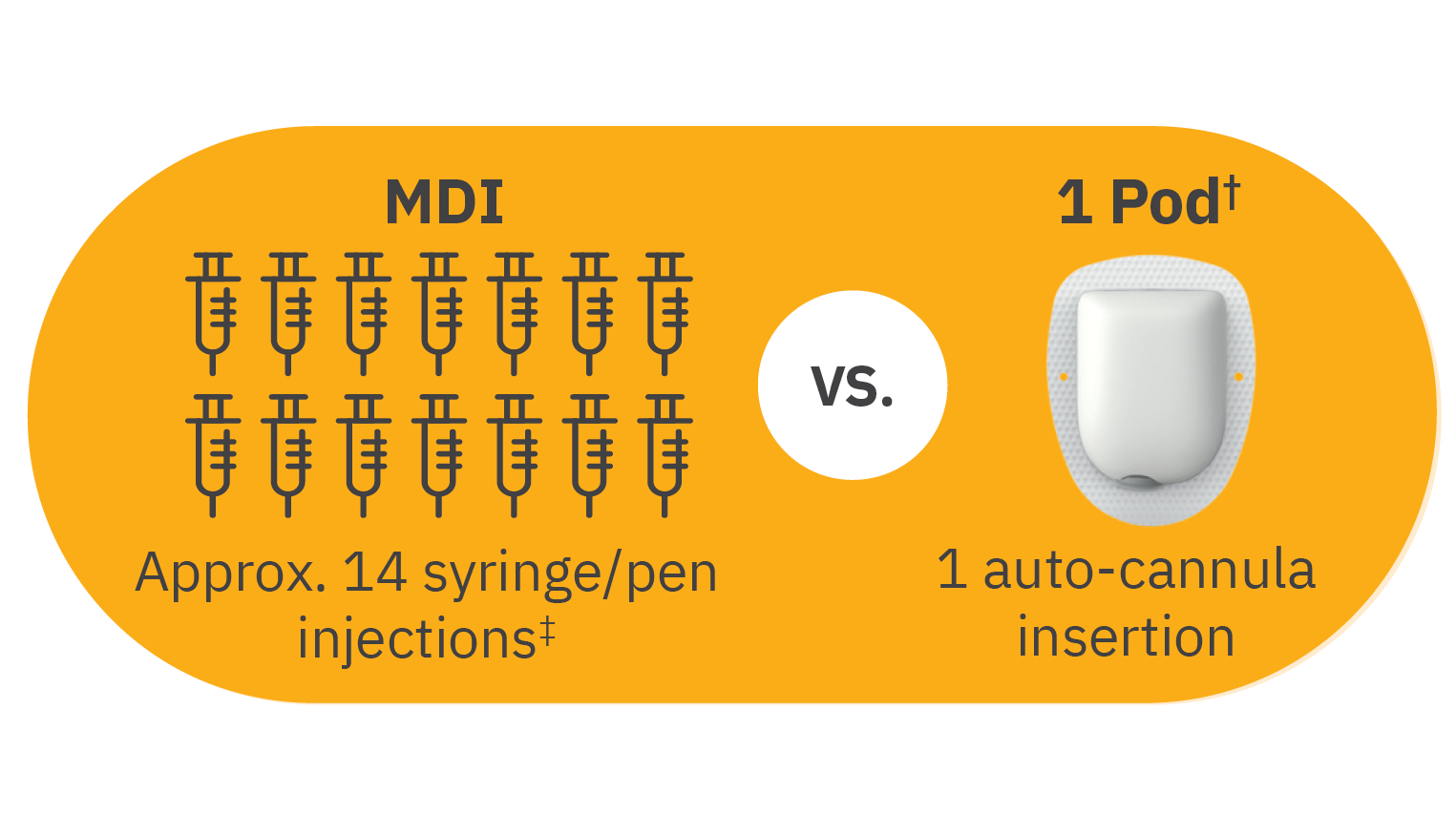
Practical benefits for your insulin dependent patients on Multiple Daily Injections (MDI)
Suitable for all ages
No need to see or touch an insertion needle
Auto-insertion feature is accurate and virtually pain free†
Worn almost anywhere that insulin would usually be injected
Get continuous insulin delivery for up to 3 days (72 hours)

Every ~3 days

Yearly
3 simple steps
1. Fill the Pod
2. Apply the Pod
3. Press Start
Please refer to the Omnipod DASH User Guide for complete safety information including indications, contraindications, warnings, cautions, and instructions.
The practical benefits of Omnipod DASH can be a life changer for your MDI patients.
Find out more about the simplicity of Omnipod DASH for your patients and download resources that you can share
Omnipod DASH is well studied in clinical and real-world settings measuring clinical efficacy and patient-reported quality of life (QOL).
Improved Glycemic Control
A simple system with demonstrated clinical results1
Real-world evidence from a large cohort of 4,738 children, adolescents, and adults with type 1 diabetes showed significant clinical improvements in A1C, and a substantial reduction in self-reported hypoglycemic events, after 3 months of Omnipod DASH use.1
Significant improvement in A1C1
Adults (n=3,341):
(≥18yrs)
Overall improvement in A1C of -0.9 (p <0.0001)%
Pediatric (n=1,397):
(<18yrs)
Overall improvement in A1C of -0.9 (p<0.0001)%
Significant reduction in self-reported hypoglycemia1
- Participants who had a lower A1C at baseline experienced the largest reduction
- Hypoglycemia events in adults reduced by more than 50% (from 2.9/week on prior therapy to 1.3/week on Omnipod DASH, p<0.0001)
- Hypoglycemia events in pediatric cohort were nearly halved (from 2.8/week on prior therapy to 1.5/week on Omnipod DASH, p<0.0001)
Total daily dose of insulin decreased1
Adults with the highest A1C at baseline (≥10.0%) saw the biggest reduction in daily amount of insulin used, -17.6 U/d (p<0.0001), while also achieving the biggest improvement in A1C (-2.9%). In the pediatric cohort, the group with the highest A1C at baseline (≥10.0%) achieved the biggest improvement in A1C (-3.5%).
Improved clinical outcomes for your type 1 diabetes patients.1
Patient-reported Quality of Life Improvement
Omnipod DASH leads to higher treatment satisfaction
First controlled, randomized study of 65 adults with type 1 diabetes in Australia assessing participantreported outcomes using validated instruments demonstrated greater treatment satisfaction at 12 weeks in the Omnipod DASH group that was sustained after 24 weeks of device use 2
- Omnipod DASH users reported higher treatment satisfaction than MDI or tubed insulin pumps amongst adults.
- Adults reported increased ease of use with Omnipod DASH compared to MDI or tubed insulin pumps.
- Omnipod DASH users reported less diabetes distress and burden compared to MDI or tubed insulin pumps amongst adults.
Significant improvements in patient-reported outcomes and A1C.
Omnipod DASH simplifies diabetes management for you and your patients!
Omnipod DASH has made a meaningful difference in peoples’ lives
Patients with high insulin sensitivity
Meet Chloé
- A spunky and smart 9-year-old who inspires others with her gymnastic skills, independence, and determination
- A1C has improved from 7.1% to 6.5%
- Fewer episodes of hypoglycemia
- It has given her more independence by allowing a 0.0 units/hr basal rate delivery and ensuring precise dosing in small increments for her high insulin sensitivity, reducing stress and anxiety for the entire family.
Chloé ◊Sponsored Podder since 2021
Kaleb ◊Sponsored Podder since 2022
Patients with an active lifestyle
Meet Kaleb
- A 20-year-old chiropractic student busy juggling schoolwork, advocacy, volunteering, and sports
- A1C has improved from 7.2% to 6.4%
- Values the flexibility to better adjust insulin doses, and the tubeless freedom and discretion of Omnipod DASH
Patients who value simplicity
Meet Cheryl
- A professional in her late 60s with a busy work schedule, who enjoys swimming* and taking care of her family
- A1C has improved from 10.1% to 7.3%
- Loves the convenience of mealtime calculations, discretion, and easy dosing with Omnipod DASH
Cheryl ◊Sponsored Podder since 2020
Insulet is here to support you and your patients
*The Pod has a waterproof IP28 rating for up to 7.6 metres (25 feet) for 60 minutes. The PDM is not waterproof.
†Omnipod User Evaluation. This validation was completed on a prior generation of the Omnipod Insulin Management System.
‡14 injections/3 days based on people with T1D on MDI taking ≥3 bolus and 1-2 basal injections/day (~4.5/day) multiplied by 3 days. Chiang JL, et al. Diabetes Care. 2014;37(7):2034-2054.1
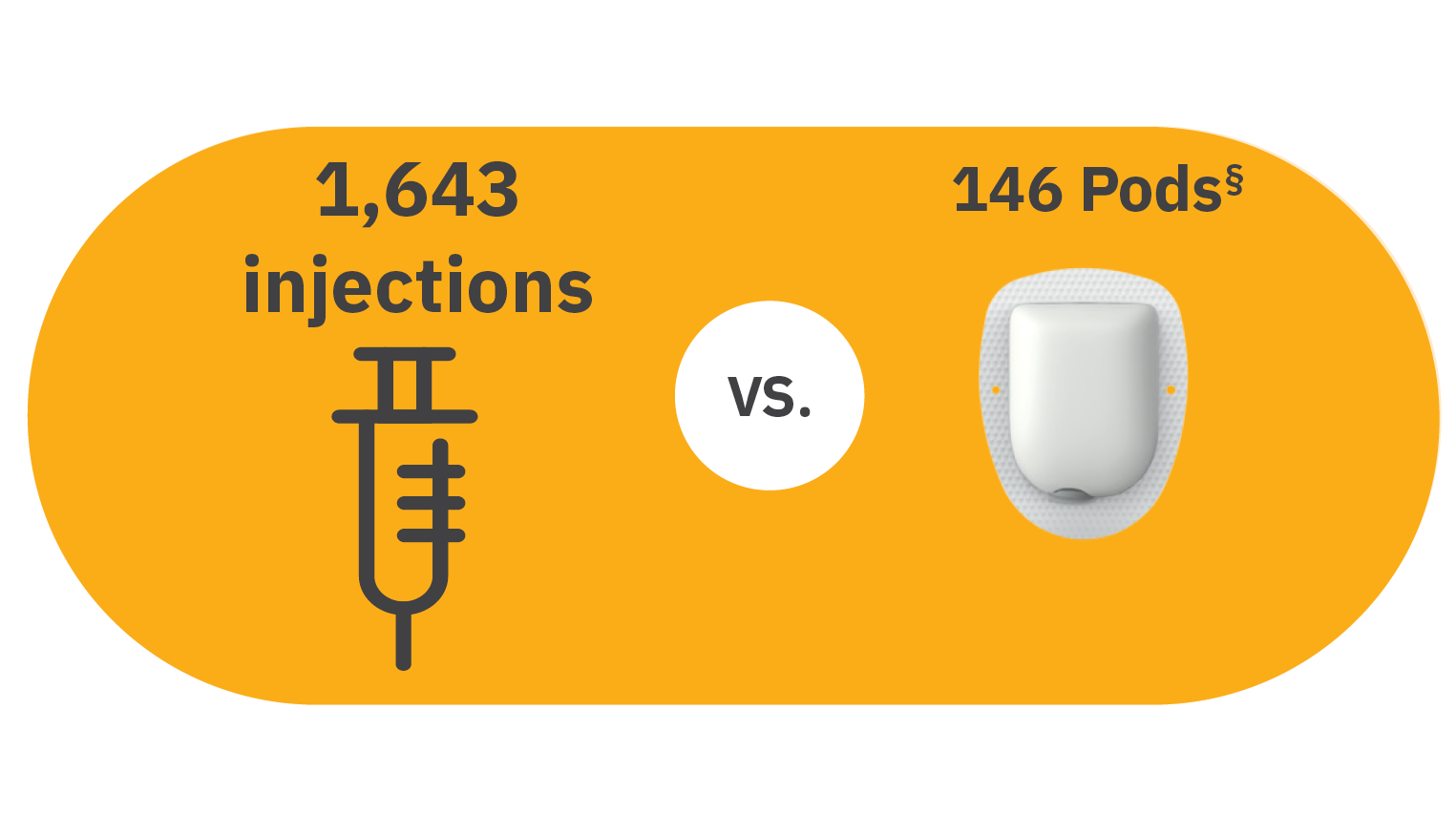
§1,643 injections in a year based on people with T1D on MDI taking ≥3 bolus and 1-2 basal injections/day (~4.5/day) multiplied by 365 days. Chiang JL, et al. Diabetes Care. 2014;37(7):2034- 2054.
¶ 146 Pods/1 year based on people changing pods every 2-3 days (on average, every 2.5 days over 365 days in a year). Omnipod. Important Safety Information. Using the Omnipod DASH System. https://www.omnipod.com/safety.
◊At the time this was written, Insulet paid a fee to engage Chloé, Kaleb, and Cheryl as content creators and has a commercial relationship with Chloé, Kaleb, and Cheryl as a Sponsored Podvocates. Patient cases are intended only for discussion about patient experiences. Patient cases are not intended for diagnosis or treatment purposes. References:
1. Aleppo G, et al. Diabetes Ther. 2023;14(3):593-610. Retrospective observational study of 3341 adults with T1D aged ≥18 years and 1397 children aged <18 years in the U.S.
2. Kong YW, et al. J Clin Endocrinol Metab. 2024;109(8):1984-1995. Randomized controlled trial of 65 adults with T1D in Australia. 3 month 1:1 randomisation to either Omnipod DASH® or usual care (MDI:60% / conventional tubed insulin pump:40%).