Omnipod® 5 Automated Insulin Delivery System
Omnipod 5 is the first wearable, tubeless, hybrid closed loop system that integrates with Dexcom G6 and G7 Sensors, for people with type 1 diabetes aged 2 years and older.
The Omnipod 5 System works with the Dexcom G6 and G7 Sensors to continuously adjust, correct, and automatically deliver basal insulin according to your patients’ needs.
Omnipod 5 is currently reimbursed in Ontario and Nova Scotia. We are actively working on public reimbursement across different provinces and territories. Please return to this webpage for updates in the future, or join the Omnipod 5 interest list for email notifications.
3 Simple Parts
Controller + Pod + Sensor
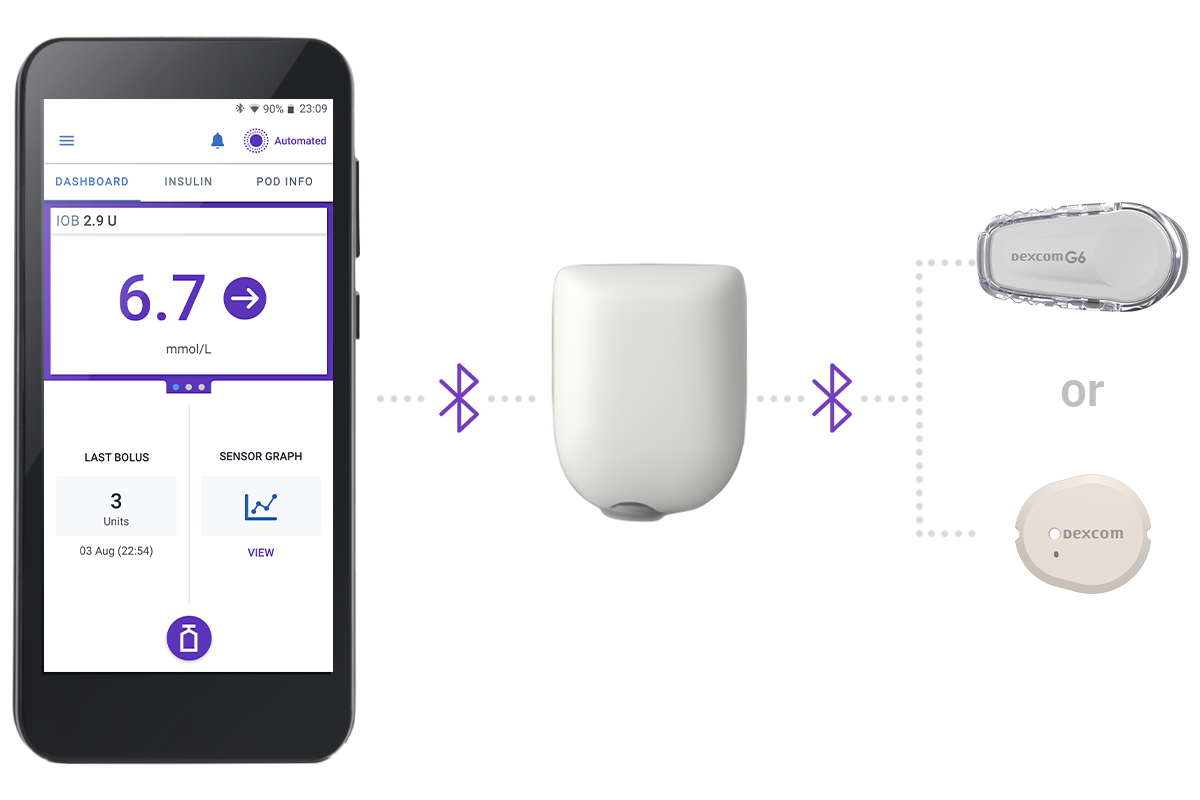

Omnipod 5 Controller
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod using Bluetooth® wireless technology.

Pod
Tubeless, wearable, and waterproof*, the Pod, with built-in SmartAdjust™ technology, sits right on your patient’s body and automatically adjusts insulin delivery for up to 3 days or 72 hours.


Sensor
Continuously sends glucose values to the Pod, so your patients can get real-time data† without the routine fingerpricks‡.


SmartAdjust™ Technology
Predicts
Glucose 60 minutes into the future
Adjusts
Insulin delivery using the selected glucose target
Delivers
Insulin doses every 5 minutes (as needed)
SmartAdjust™ technology is at the heart of Omnipod 5, proactively managing insulin delivery every 5 minutes. It automatically increases, decreases or pauses insulin delivery to your patient’s personal needs which may help to prevent against highs and lows.1
Time in range
76% of time in range for adults and adolescents (target 6.1 mmol/L) 68% of total time in range for children (2-13.9 years)1,2
HbA1c
Significantly reduced by 0.5% in very young children (2.0-5.9 years), 0.7% in children (6-13.9 years) and 0.4% in adults and adolescents (14-70 years)1,2
Hyperglycemia
Time spent in hyperglycemia in children and 24% in adults and adolescents1
Hypoglycemia
60% reduction in time in hypoglycemia overnight and 46% overall in adults and adolescents1
Safety: There were 3 severe hypoglycaemia and 1 DKA events during the pivotal study. These events were not related to automated insulin delivery malfunction.1
Omnipod 5 improved users’ quality of life in clinical studies3,4
- Parents of children using Omnipod 5 reported improvements in quality of life, sleep quality, confidence avoiding hypoglycemia, and mental well-being
- Adults using Omnipod reported improvements in diabetes distress, stress eating, confidence avoiding hypoglycemia, and mental well-being
†Compatible with the Dexcom G6 and Dexcom G7 Sensors. Sensor is required for Automated Mode. Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription. The Dexcom Sensors must be used with the respective Dexcom mobile app. The Dexcom receiver is not compatible. Devices compatible with Dexcom apps can be found at https://www.dexcom.com/compatibility.
‡Routine fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
1. Brown SA, et al. Diabetes Care. 2021;44(7):1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, p<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, p<0.0001; 2.51% vs. 1.78, p=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, p<0.0001; 2.13% vs. 1.98%, p=0.2545, respectively. Mean time >180 mg/dL or >10 mmol/L overall in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 32.4% vs. 24.7%; 45.3% vs. 30.2%, p<0.0001, respectively. Mean time <70 mg/dL or <3.9 mmol/L in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 2.9% vs. 1.3%, p<0.0001; 2.2% vs. 1.8%, p=0.8153, respectively. Results measured by CGM.
2. Sherr JL, et al. Diabetes Care. 2022;45(8):1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, p=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, p=0.0799.
3. Hood KK et al. Diabetes Obes Metab. 2024. Study of 81 children aged 6-11.9 with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean caregiver self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.34 vs 3.59, p-value <0.0001, respectively. During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively. During the Omnipod 5 pivotal trial, adults aged 18-70 (N=115) experienced an improved diabetes distress survey score after 3 months of AID use: mean: 1.48 vs. 1.64 (p< 0.001). During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively. During the Omnipod 5 pivotal trial, parents of children 6-11.9 years (N=82) experienced an improvement in sleep quality survey score after 3 months of Omnipod 5 use compared to standard therapy: mean PSQI Overall Sleep Quality Subscore = 0.70 vs 1.12, respectively.
4. Polonsky WH et al. Diabetes Res Clin Pract 2022;190:109998. Study of 115 adults aged 18-70 years with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.52 vs 3.65, p-value = 0.0002, respectively. Adults aged 18-70 (N=115) experienced reduction in eating distress score in T1-DDS survey after 3 months of Omnipod 5 use vs baseline: 1.73 vs. 1.97, (p=0.0003), respectively.