Simplify diabetes management for your patients
Omnipod® provides a simple, effective alternative to both multiple daily injections (MDI) and tubed pumps for patients requiring insulin.
Healthcare providers (HCPs) like you who care for people with diabetes are talking with more and more patients who are interested in the benefits of an insulin pump. Among Canadian HCPs, complexity is the #1 reason that pumps are not recommended for some patients.1
Pod Therapy is something you might consider instead. And now, we are proud to introduce our newest Omnipod 5 Automated Insulin Delivery system!
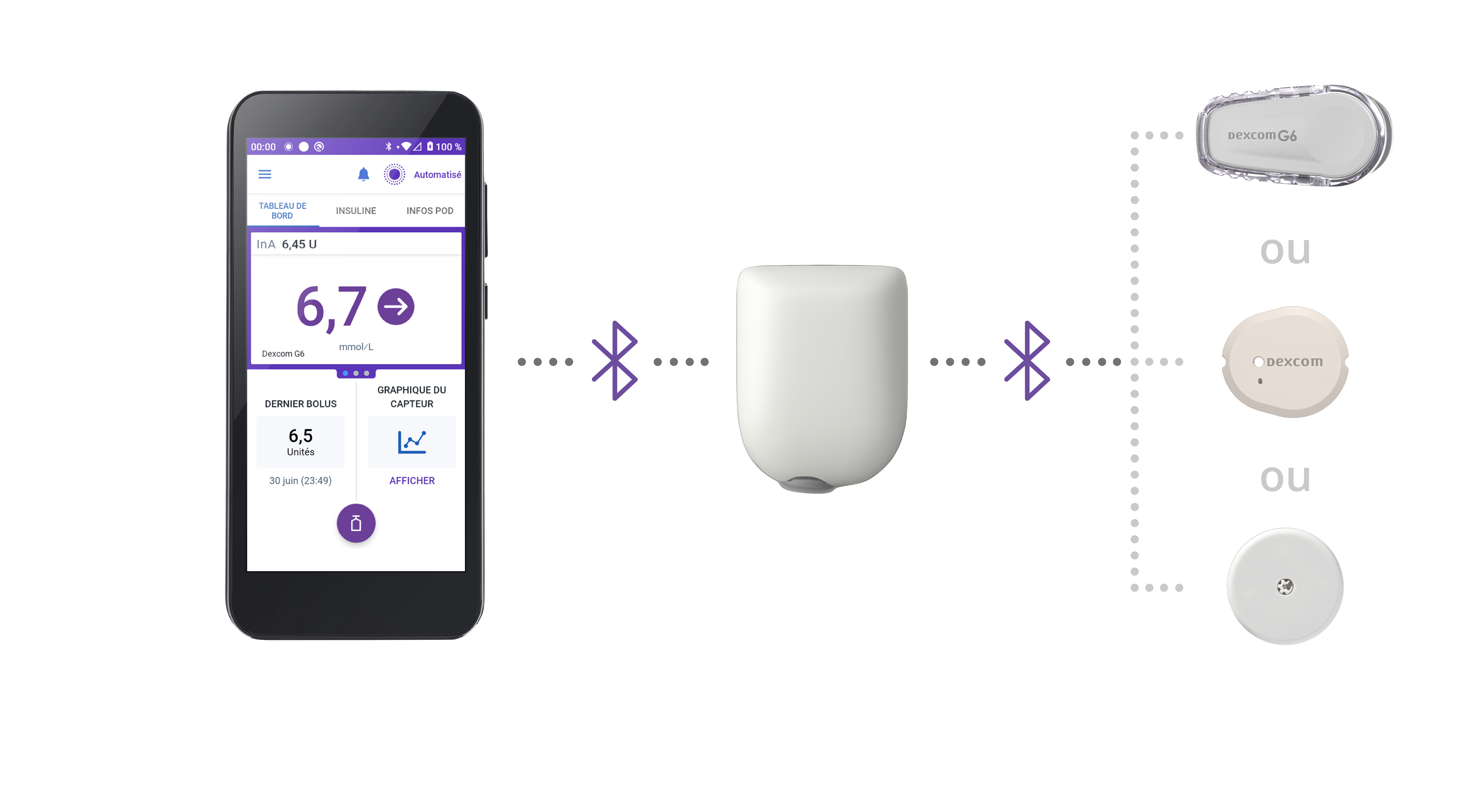
It is the first wearable, tubeless, waterproof*, hybrid closed loop system that integrates with Dexcom G6 and G7 Sensors, for your patients with type 1 diabetes (T1D) aged 2 years and older.
- Your patients can experience the freedom of automated insulin delivery
- SmartAdjustTM Technology adjusts basal insulin delivery, every 5 minutes†
- Improved HbA1c and time in range across age groups vs prior therapy, while time in hypoglycemia remained low2,3
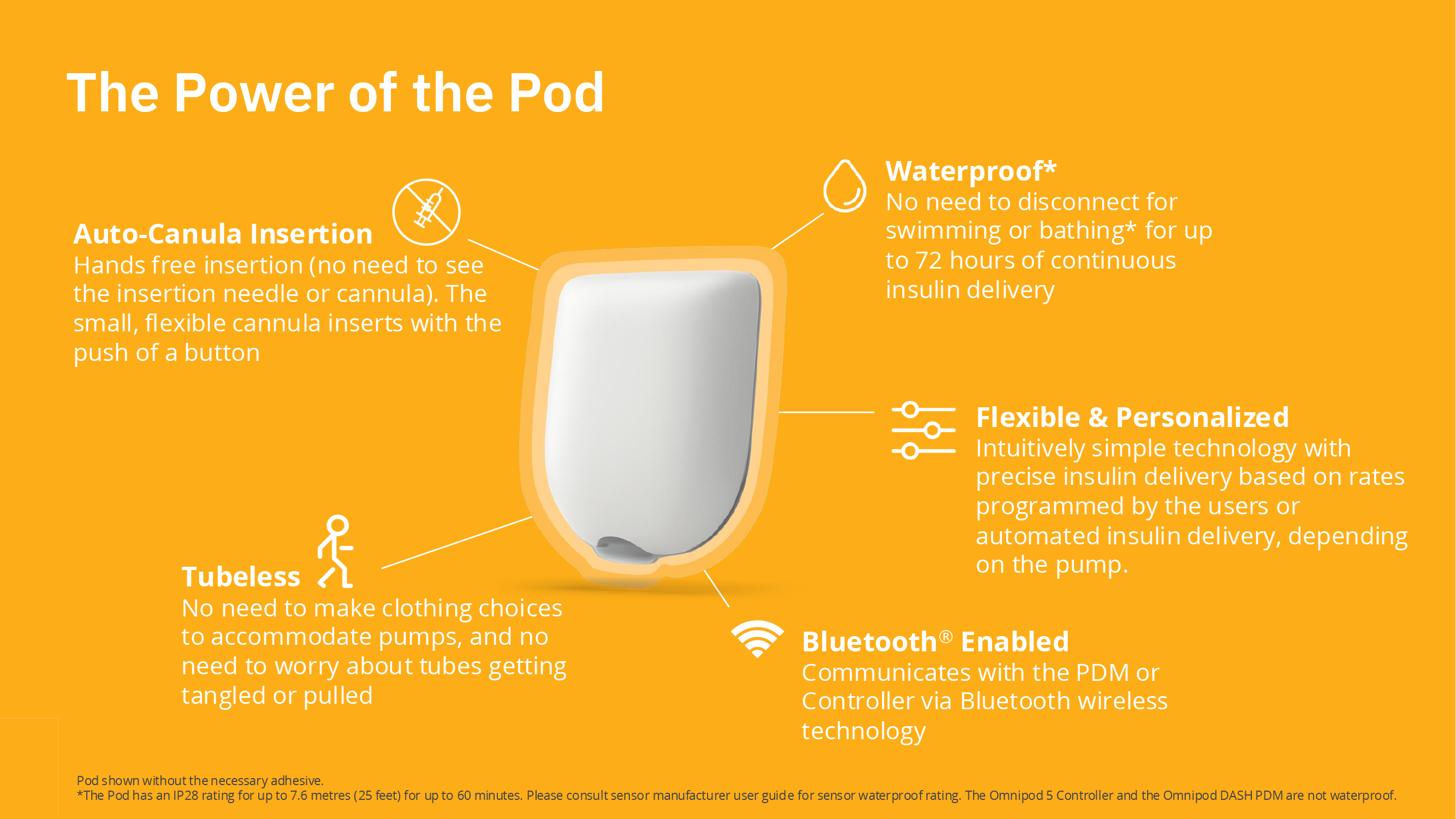

Auto-Cannula Insertion
Hands free insertion (no need to see the insertion needle or cannula). The small, flexible cannula inserts with the push of a button.
Tubeless
No need to make clothing choices to accommodate pumps, and no need to worry about tubes getting tangled or pulled.
Waterproof
No need to disconnect for swimming or bathing*
Flexible & Personalized
Intuitively simple technology with precise insulin delivery based on the set and variable rates programmed by the user's Bluetooth®
Omnipod® 5 Algorithm Explained
SmartAdjust™ Technology
SmartAdjust™ Technology automatically increases, decreases, or pauses insulin delivery, every five minutes, to your personal needs which may help to protect against highs and lows.‡ 2,3 Watch this video to learn more.
Omnipod Products
Omnipod 5
Automated Insulin Management System


First and only tubeless, waterproof*, and automated insulin delivery that works with the leading sensor brands† and proactively helps your patients correct highs while protecting from lows2,3
Omnipod DASH®
Insulin Management System


The discreet, tubeless, and waterproof* way to deliver insulin that is easy-to-use and offers hand-on control for patients that are not comfortable with automation or new technology
No multiple daily injections, no pens
Give your patients the benefits of tubeless freedom and more
- Simple to set up and train patients
- Quick access to patient data from anywhere with Glooko®
- Intuitive screen navigation and quick treatment modifications
- Virtually pain-free automated cannula insertion
Omnipod is the right choice for patients for different reasons.
See which of your patients might be ready to switch to Pod Therapy
Have a patient ready to experience the tubeless freedom of Omnipod?
*The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Omnipod 5 Controller and the Omnipod DASH PDM are not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
†A compatible sensor is required for Automated Mode. Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription. The Dexcom Sensors must be used with the Dexcom mobile app. The Dexcom receiver is not compatible. Devices compatible with Dexcom apps can be found at dexcom.com/compatibility.
‡As with all hybrid closed loop systems, users still need to bolus for meals and corrections. With Omnipod 5, this is done with the Omnipod 5 Controller.
References:
1. Data on File, Insulet Canada, 2022.
2. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), p<0.0001). Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, p<0.0001, respectively. Mean time in hypoglycaemic range in adults/adolescents (<3.9 mmol/L or <70mg/dL as measured by CGM) as measured by CGM: ST = 1.89%, 3-mo Omnipod 5 = 1.32%, p<0.0001. Mean time in hypoglycaemic range in children (<3.9 mmol/L or <70mg/dL as measured by CGM): ST = 2.21%, 3-mo Omnipod 5 = 1.78%, p<0.0456.
3. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, p<0.0001. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, p<0.0001, respectively. Mean time in hypoglycaemic range (<3.9mmol/L or <70mg/dL as measured by CGM) ST = 3.43% vs Omnipod 5: 2.46%, p<0.0001.