Omnipod® for children
We understand that caring for a child with type 1 diabetes isn’t easy. You want an insulin delivery method that can meet your child’s evolving health needs, while also granting them the freedom of being a child.
Omnipod works with you and your little one to do just that. With no multiple daily injections and no tubes, Pod Therapy is insulin pump therapy, simplified. It’s a system you can trust.
The Omnipod 5 is our Automated Insulin Delivery (AID) System. It’s the only tubeless AID System that works with the leading Sensor brands, creating a fully waterproof† and fully wearable AID System compatible with the Dexcom G6 Sensor, Dexcom G7 Sensor or FreeStyle Libre 2 Plus Sensor (launch timings might differ between Sensors compatibility).
With no finger prick testing‡ and no need to disconnect**, your child is free to live life with fewer interruptions. Meanwhile, you can share the load with a system that’s always adjusting* so you don’t have to.
The benefits of Omnipod
Freedom to play, freedom to learn, freedom to be a child!
No multiple daily injections, and no tubes: Each tubeless, wearable Pod delivers insulin for up to three days (72 hours). Your child can go to a dance class or play video games with fewer interruptions.
Waterproof†: The Pod can be worn while swimming, bathing, or showering.
Discreet: Small enough to be worn under clothing, with no tubes hanging loose.
Accurate: Precise dosing with 0.05 unit increments gives you greater control around mealtimes.
All of this could mean less diabetes disruption for your family, greater peace of mind for you and more independence for your little one.
How does the Omnipod work?
Pod Therapy from Omnipod is a tubeless insulin delivery system designed for people with type 1 diabetes aged 2 years and older.
Pod Therapy replaces the need for multiple daily injections by delivering small, customisable doses of insulin to your child’s body throughout the day, through a wearable and waterproof† patch pump called a Pod.
Omnipod differs from other insulin pump products with its innovative, compact, and tubeless design, made to fit around you and your child’s busy lives.
Meet our Tubeless, wearable Omnipod lineup
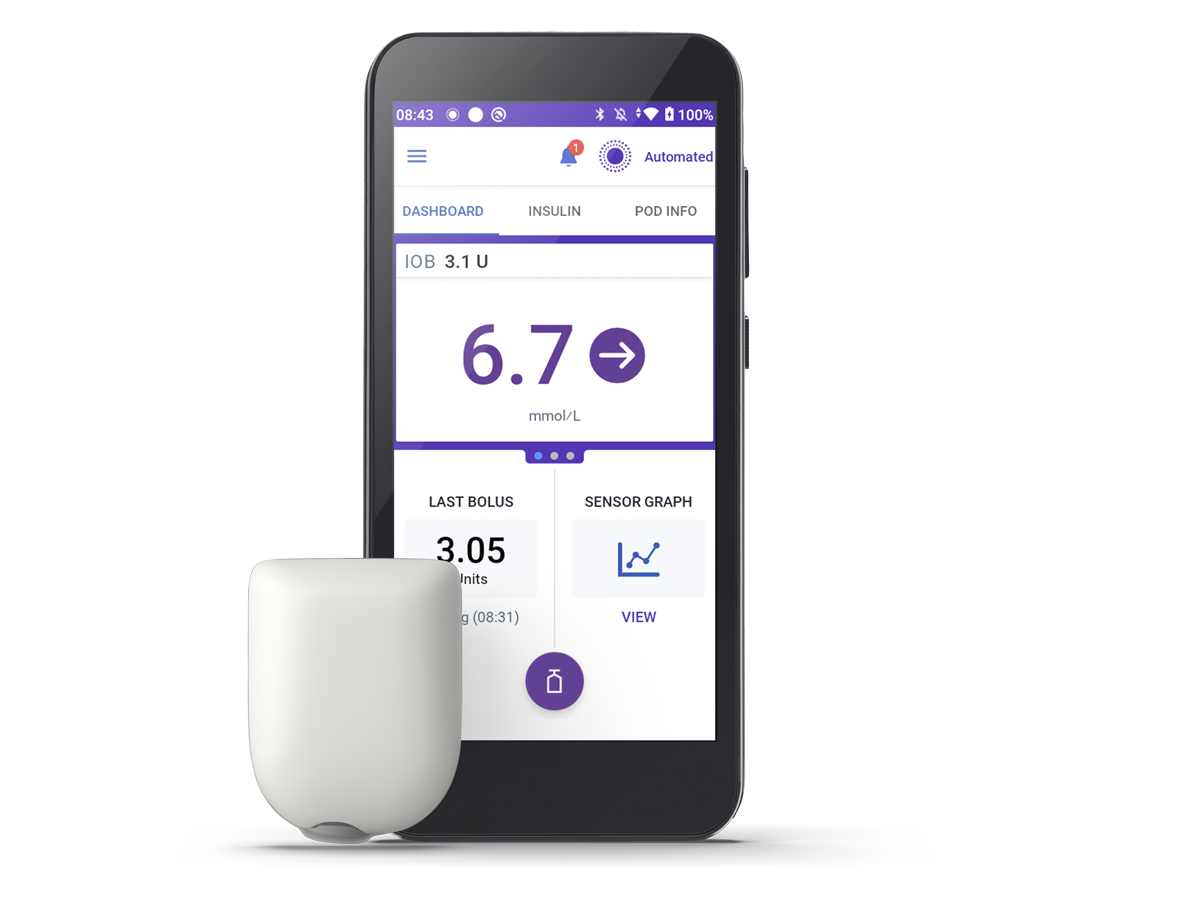

Omnipod 5 Automated Insulin Delivery System
Omnipod 5 is the first and only tubeless automated insulin delivery system that works with the leading Sensor brands*.
Compatible with the Dexcom G6 Sensor, Dexcom G7 Sensor and the FreeStyle Libre 2 Plus Sensor, Omnipod 5 makes automated insulin adjustments every five minutes based on CGM readings, to help keep your child in range1,2.


Omnipod DASH® Insulin Management System
Simplify Life® with Omnipod DASH. Place the Pod on your child’s body almost anywhere you would inject insulin for up to three days (72 hours) of precise, continuous insulin delivery.
Discreetly and conveniently adjust your child’s insulin doses with a few finger taps wherever you are‡, without any insulin pump tubing.


Don’t let the terminology hold you back!
There’s never been a better time to embrace diabetes technology with Omnipod, but the terminology can be confusing.
Pod University is our library of resources that cuts through the jargon. You can learn about different diabetes devices at your own pace, and explore the potential benefits for your child.
Request your free demo Pod◊ today!
The Pod Experience Kit◊ contains a non-functioning Pod for your child to try. It’s the same size and weight of a real Pod, designed to demonstrate what it feels like for your child to wear the Pod and to get a sense of how discreet it can be.
* Requires Dexcom G6, Dexcom G7 or FreeStyle Libre 2 Plus Sensor. Bolus for meals and corrections are still needed.
** The Pod will need to be disconnected for MRI scans
‡ Finger pricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, P<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, P<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), P<0.0001). Mean time in hypoglycaemic range in adults/adolescents (<3.9 mmol/L or <70mg/dL as measured by CGM) as measured by CGM: ST = 1.89%, 3-mo Omnipod 5 = 1.32%, P<0.0001. Mean time in hypoglycaemic range in children (<3.9 mmol/L or <70mg/dL as measured by CGM): ST = 2.21%, 3-mo Omnipod 5 = 1.78%, P<0.0456.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (P<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, P<0.0001. Mean time in hypoglycaemic range (<3.9mmol/L or <70mg/dL as measured by CGM) ST = 3.43% vs Omnipod 5: 2.46%, P<0.0001.
#The demo Pod is a needle-free Pod that does not deliver insulin. The PDM/Controller is not included.