Simplify Life with Omnipod® 5


No multiple daily injections or finger prick testing‡. Omnipod 5 proactively helps to correct highs and protect from lows1,2 –simplify your diabetes management.
Get to know the Omnipod 5 System


The Pod
Tubeless, wearable, and waterproof†, each Pod sits comfortably on the body for up to 3 days (72 hours), automatically adjusting insulin delivery thanks to its built-in SmartAdjust™ technology.


The Controller
Take charge of your diabetes with the handheld Omnipod 5 Controller, which connects seamlessly to your Pod via Bluetooth® for discreet, wireless control.


The Sensor
Your choice of sensor continuously sends glucose values to the Pod, giving you real-time readings without the hassle of finger pricks‡. The Pod and sensor need to be in ‘line of sight’ to stay in Automated Mode. Placing them on the same side of the body allows for best communication between the devices.
3 simple parts. One continuous loop of communication.
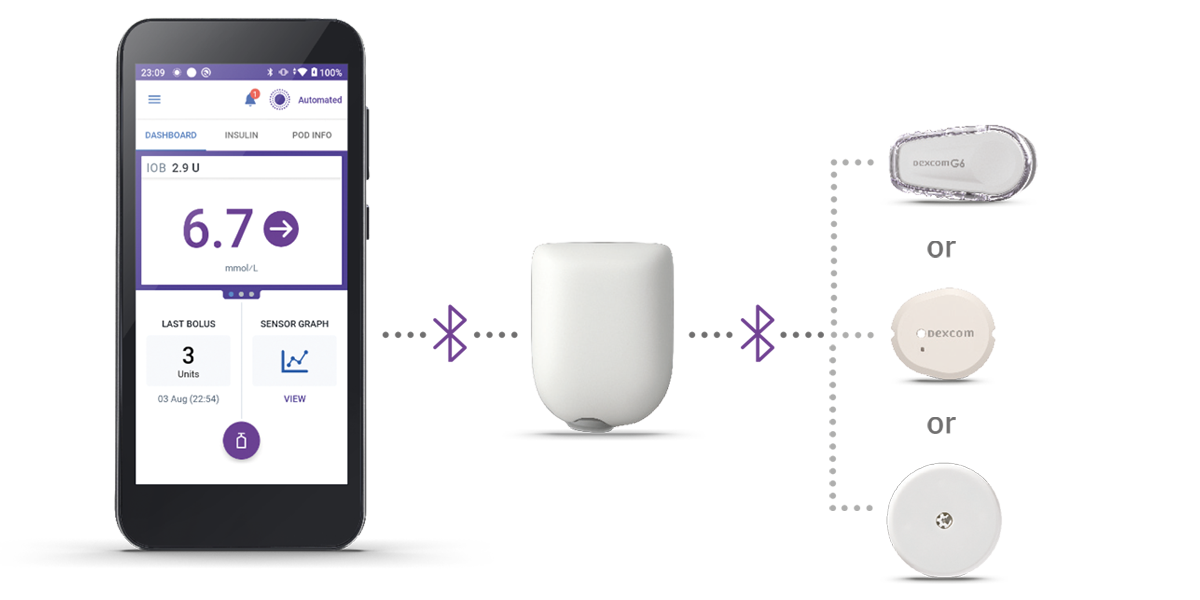

When paired with a compatible Continuous Glucose Monitor (CGM) sensor, Omnipod 5 can give constant and automatic insulin delivery to keep you in range, day and night1,2. Omnipod 5 is compatible with Dexcom G6, Dexcom G7, and Freestyle Libre Plus 2 Sensor.
Here’s what our Podders have to say about Omnipod®:
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
Get started with one of these options


Speak to an Omnipod Specialist
Still have more questions about the Omnipod 5? Enter your information below and one of our Omnipod® Specialists will call you in 24-48 hours for a one-to-one chat.


Pod Experience Kit
The Pod Experience Kit* contains a real-size, real weight demo Pod*, without the insulin. It’s designed to give you an idea of what it feels like to wear a Pod, and get a sense of how discreet it can be.
Want to know more about Omnipod?
To learn more about Omnipod 5, check out our library of resources.
‡Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
†The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Controller is not waterproof.
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70- 180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, P<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, P<0.0001. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, P<0.0001, respectively. Mean time in hypoglycaemic range in adults/adolescents (<3.9 mmol/L or <70mg/dL as measured by CGM) as measured by CGM: ST = 1.89%, 3- mo Omnipod 5 = 1.32%, P<0.0001. Mean time in hypoglycaemic range in children (<3.9 mmol/L or <70mg/dL as measured by CGM): ST = 2.21%, 3-mo Omnipod 5 = 1.78%, P<0.0456.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3- month AID phase with Omnipod 5 system. Mean time in range (3.9-10.0 mmol/L or 70- 180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, P<0.0001. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9mmol/L or <70mg/dL as measured by CGM) ST = 3.43% vs Omnipod 5: 2.46%, P<0.0001.
The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin.
The Omnipod 5 System is intended to operate as an automated insulin delivery system when used with compatible Continuous Glucose Monitors (CGM). When in automated mode, the Omnipod 5 system is designed to assist people with type 1 diabetes in achieving glycaemic targets set by their healthcare providers. It is intended to modulate (increase, decrease or pause) insulin delivery to operate within predefined threshold values using current and predicted CGM values to maintain blood glucose at variable target glucose levels, thereby reducing glucose variability. This reduction in variability is intended to lead to a reduction in the frequency, severity, and duration of both hyperglycaemia and hypoglycaemia.
The Omnipod 5 System can also operate in a manual mode that delivers insulin at set or manually adjusted rates.
The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is indicated for use with NovoLog®/NovoRapid®, Humalog® / Liprolog®, Admelog® / Insulin lispro Sanofi®, Trurapi® / Insulin aspart Sanofi®, and Kirsty® U-100 insulin.
Warning: SmartAdjust™ technology should NOT be used by anyone under the age of 2 years old. SmartAdjust™ technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.
The Omnipod® 5 System is NOT recommended for people who are unable to monitor glucose as recommended by their healthcare provider, are unable to maintain contact with their healthcare provider, are unable to use the Omnipod® 5 System according to instructions, are taking hydroxyurea and using a Dexcom Sensor as it could lead to falsely elevated CGM values and result in over-delivery of insulin that can lead to severe hypoglycaemia, and do NOT have adequate hearing and/or vision to allow recognition of all functions of the Omnipod® 5 System, including alerts, alarms, and reminders. Device components including the Pod, CGM transmitter, and CGM sensor must be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment. In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to MRI, CT, or diathermy treatment can damage the components.
Warning: DO NOT start to use the Omnipod® 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycaemia or hyperglycaemia.
