Omnipod® for Children
We understand that caring for a child with type 1 diabetes isn’t easy. You want an insulin delivery method that can meet your child’s evolving health needs, while also granting them the freedom of being a child.
Omnipod works with you and your little one to do just that. With no multiple daily injections and no tubes, Pod Therapy is insulin pump therapy, simplified.
Think about a life beyond MDI!
Omnipod 5 users agree that it is easy to use*
The benefits of Omnipod
Freedom to play, freedom to learn, freedom to be a kid!
No multiple daily injections, and no tubes: Each tubeless, wearable Pod delivers insulin for up to 3 days (72 hours). Your child can go to a dance class or play video games with fewer interruptions.
Waterproof†: The Pod can be worn while swimming, bathing, or showering.
Discreet: Small enough to be worn under clothing, with no tubes hanging loose.
Accurate: Precise dosing with 0.05 unit increments gives you greater control around mealtimes.
All of this could mean less diabetes disruption for your family, greater peace of mind for you - and more independence for your little one.
How does the Omnipod work?
Pod Therapy from Omnipod is a tubeless insulin delivery system designed for people with type 1 diabetes ages 2 years and older.
Pod Therapy replaces the need for multiple daily injections by delivering small, customizable doses of insulin to your child’s body steadily throughout the day, through a wearable and waterproof† patch pump called a Pod.
Omnipod differs from other insulin pump products with its innovative, compact, and tubeless design, made to fit around you and your child’s busy lives.
Meet our tubeless, wearable Omnipod line-up
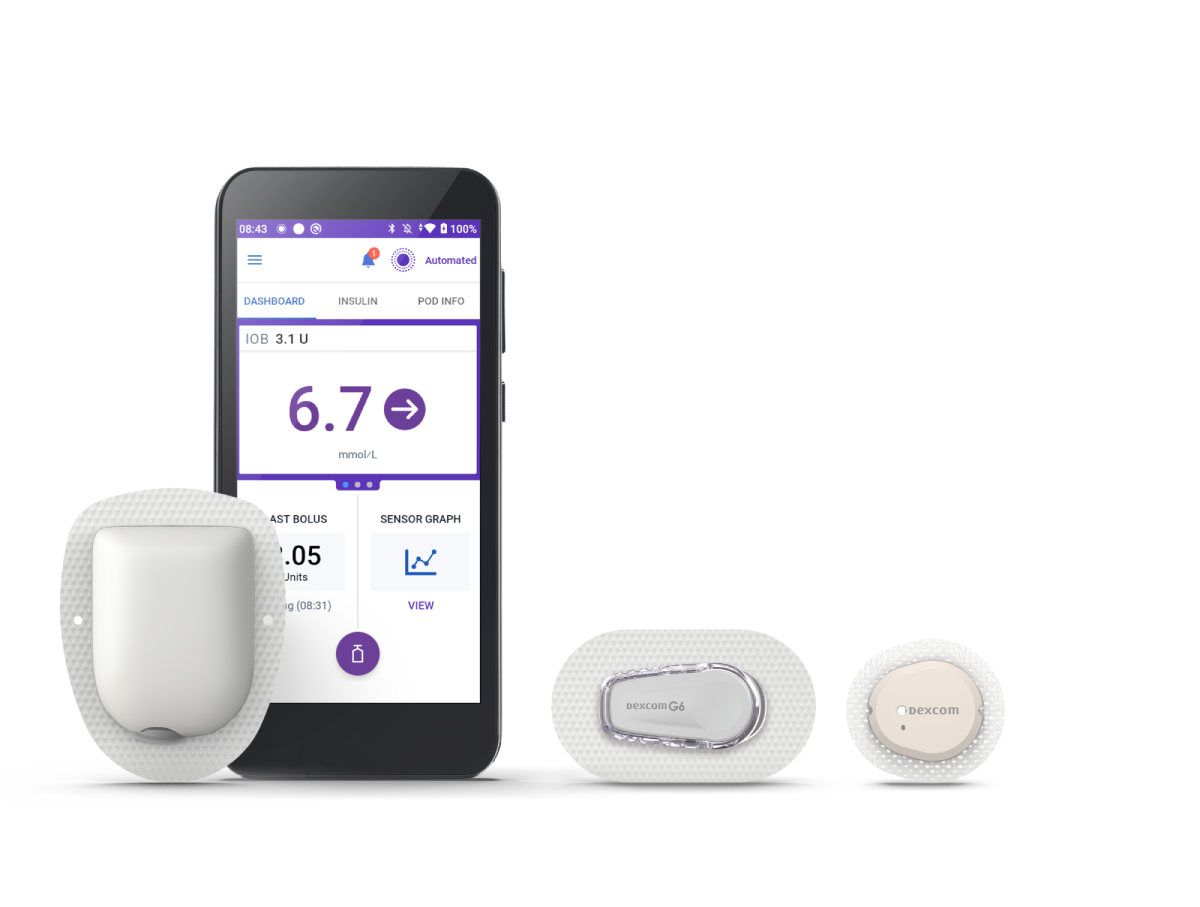

Omnipod 5 Automated Insulin Delivery System
Omnipod 5 is the first and only tubeless automated insulin delivery system that works with Dexcom G6 and G7 Sensors.‡
Omnipod 5 makes automated basal insulin adjustments every five minutes to help keep your child in range.1,2


Omnipod DASH Insulin Management System
Simplify Life® with Omnipod DASH. Place the Pod on your child’s body almost anywhere you would inject insulin for up to 3 days (72 hours) of precise, continuous insulin delivery.
Discreetly and conveniently adjust your child’s insulin doses with a few finger taps wherever you are§, without any insulin pump tubing.
Want to learn more about Omnipod?
We offer free Pod Therapy 101 live webinars, where you can explore the following topics and participate in a Q & A session to address your questions and concerns:
- What is Pod Therapy and how is it different than insulin pump therapy?
- An overview of Omnipod 5
- Deeper dive into the Omnipod 5 SmartAdjust™ technology
- An overview of Omnipod DASH
- Next steps to get started on Omnipod
- Insulet support programs and resources for new and current patients
- Live Q & A with an Omnipod representative
Request your free demo Pod¶ today!
The Pod Experience Kit¶ contains a non-functioning Pod for your child to try. It’s the same size and weight of a real Pod, designed to demonstrate what it feels like for your child to wear the Pod and to get a sense of how discreet and comfortable it can be.
*Insulet Data on File. OP5-003 Clinical Study Report. 2024. A 13-week randomized controlled trial conducted among 194 adults (age 18-70 years) with type 1 diabetes in France and the U.S., comparing the safety and effectiveness of the Omnipod 5 Automated Insulin Delivery (AID) System versus conventional non-AID pump therapy and CGM (control). At 13-weeks of Omnipod 5 use, 130 participants were asked via a System Usability Survey (SUS) if the system was easy to use. 71.5% of participants strongly agreed and 19.2% agreed.
†The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. Please consult sensor manufacturer user guide for sensor waterproof rating. The Omnipod DASH PDM and Omnipod 5 Controller are not waterproof.
‡Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription. Sensors must be used with compatible apps on a supported smartphone.
§At start-up, the Pod and Controller or PDM must be adjacent and touching. During normal operation, the Personal Diabetes Manager must be within 1.5 metres of the Pod.
¶The PEK includes a non-functioning, needle-free Pod which allows you to experience how it feels to wear a Pod. The demo Pod is for demonstrative purposes only and is not able to deliver insulin. This PEK is available only to people with type 1 diabetes who are on a current insulin treatment (e.g., multiple daily injections or insulin pump therapy). People with type 2 diabetes are not eligible to receive a PEK unless they reside in British Columbia. The PEK does not include a physical Omnipod DASH PDM or the Omnipod 5 Controller.
1. Study in 240 people with T1D aged 6 - 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in adults/adolescents and children, standard therapy vs. 3-month Omnipod 5: 32.4% vs. 24.7%; 45.3% vs. 30.2%. Median time with low blood glucose in adults/adolescents and children, standard therapy vs. 3-mo Omnipod 5: 2.0% vs. 1.1%; 1.4% vs. 1.5%. Brown SA, et al. Diabetes Care (2021).
2. Study in 80 people with T1D aged 2 - 5.9 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose (>180mg/dL) from CGM in standard therapy vs Omnipod 5 = 39.4% vs. 29.5%. Average time with low blood glucose (<70mg/dL) from CGM in standard therapy vs Omnipod 5 = 3.41% vs. 2.13%. Sherr JL, et al. Diabetes Care (2022).