Is Omnipod® Right for Me?
A bit about insulin pump therapy
An insulin pump is a device that delivers insulin in place of multiple daily injections.
A continuous amount of rapid acting insulin is delivered 24 hours a day through a cannula placed under the skin. This is known as basal insulin, but it’s sometimes called background insulin.
With automated insulin delivery systems, these basal doses are automatically calculated and delivered every five minutes - which can mean less diabetes ‘work’ for you.
Additional insulin is delivered for meals and blood glucose corrections - these are known as bolus doses.
There is more than one type of insulin pump.
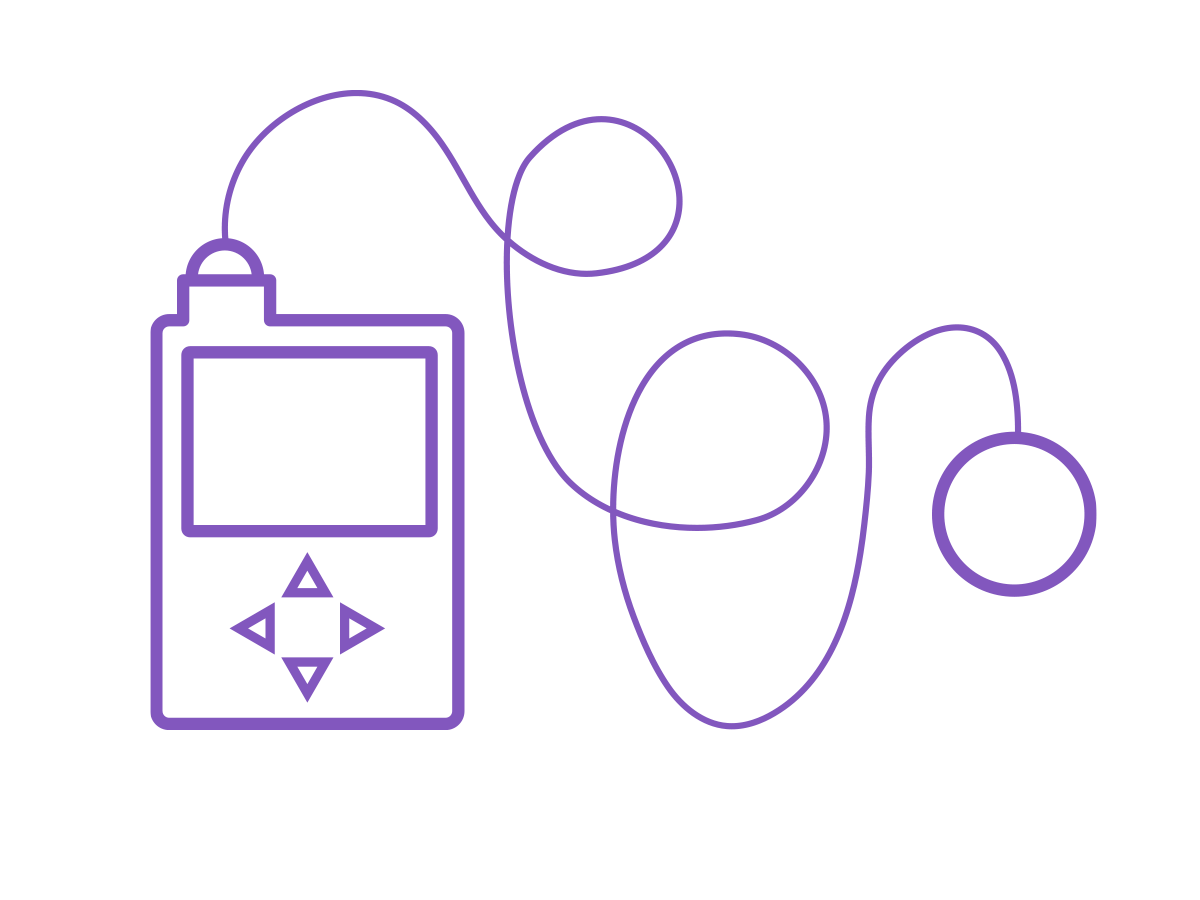

Tethered pumps
A tethered pump is sometimes called a tubed pump. The pump itself contains the insulin, and this is delivered to the body through a tube via an infusion set with a cannula. The tubing allows the pump to be kept in your pocket, or clipped to a belt or bra.
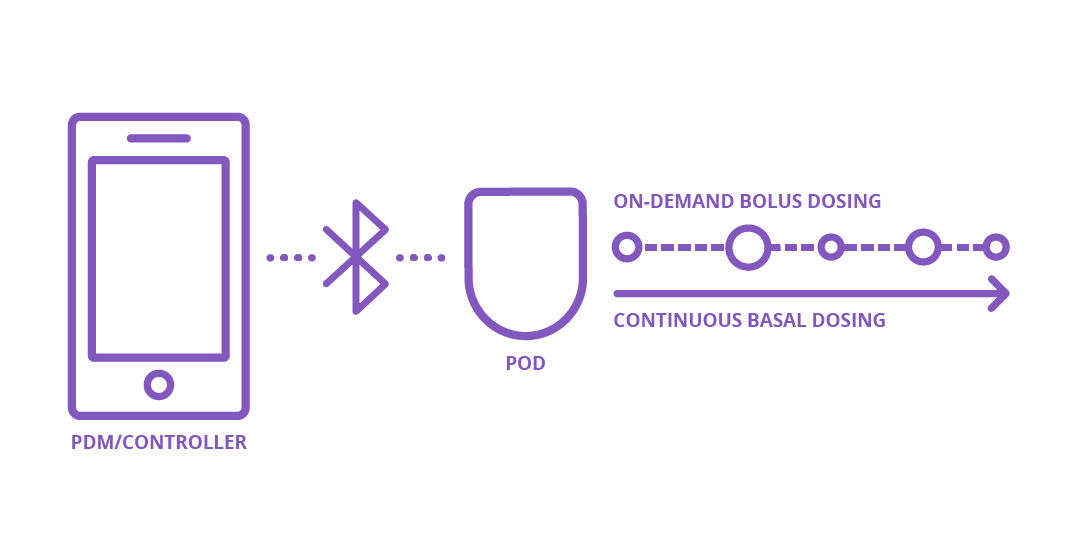

Patch pumps
A tubeless pump is sometimes called a patch pump. The part that contains the insulin sticks directly to the skin with adhesive, so there is no connecting tube. Insulin dosing is remotely programmed from a separate device, a bit like a remote control, and delivered to the body through a short cannula inserted directly under the skin. Omnipod is a tubeless pump!
There is no exaggeration on my part though, when I say Omnipod® has proven life changing ever since I made the switch.
Will G.
Podder® Since 2021
Why Omnipod?
Omnipod was created out of a father’s mission to free his son from the burden of multiple daily injections. Our mission to simplify life for those living with type 1 diabetes remains.
From parents frustrated by the need to deliver frequent injections, to adults who’ve written off insulin pumps altogether, Omnipod can simplify life with up to three days (up to 72 hours) of continuous, tubeless insulin delivery, with no need for multiple daily injections.
- Discreet: The Pod is small enough to be worn under clothing. Placed directly on the body with adhesive, there’s no tubing to worry about.
- Convenient: Deliver bolus insulin with just a few fingertaps using the wireless Controller (Omnipod® 5) or Personal Diabetes Manager (Omnipod DASH®).
- Simplified: The built-in Bolus Calculator reduces mealtime maths.
- Waterproof‡: The Pod can be worn in the shower, swimming pool or sea.
We have two systems. Our Omnipod DASH® system and our next generation automated insulin delivery (AID) system, Omnipod® 5. Omnipod 5 is the first and only tubeless automated insulin delivery system that works with the leading sensor brands*.
We have two systems: the Omnipod DASH® System and our next generation automated insulin delivery (AID) system, Omnipod® 5.
Omnipod 5 is the first and only tubeless automated insulin delivery system that works with the leading sensor brands*.
Compatible with your choice of the Dexcom G6 Sensor, Dexcom G7 Sensor or FreeStyle Libre 2 Plus Sensor (launch timings to be confirmed), you can get started with all the benefits of automated insulin delivery without the hassle of having to switch your continuous glucose monitor.
Pod Therapy is insulin pump therapy, but not as you know it!
* Automated Mode requires Dexcom G6, Dexcom G7 or FreeStyle Libre 2 Plus sensor. Boluses for meals and corrections are still needed. Sensor requires a separate prescription and is sold separately.
Meet our tubeless, wearable Omnipod lineup
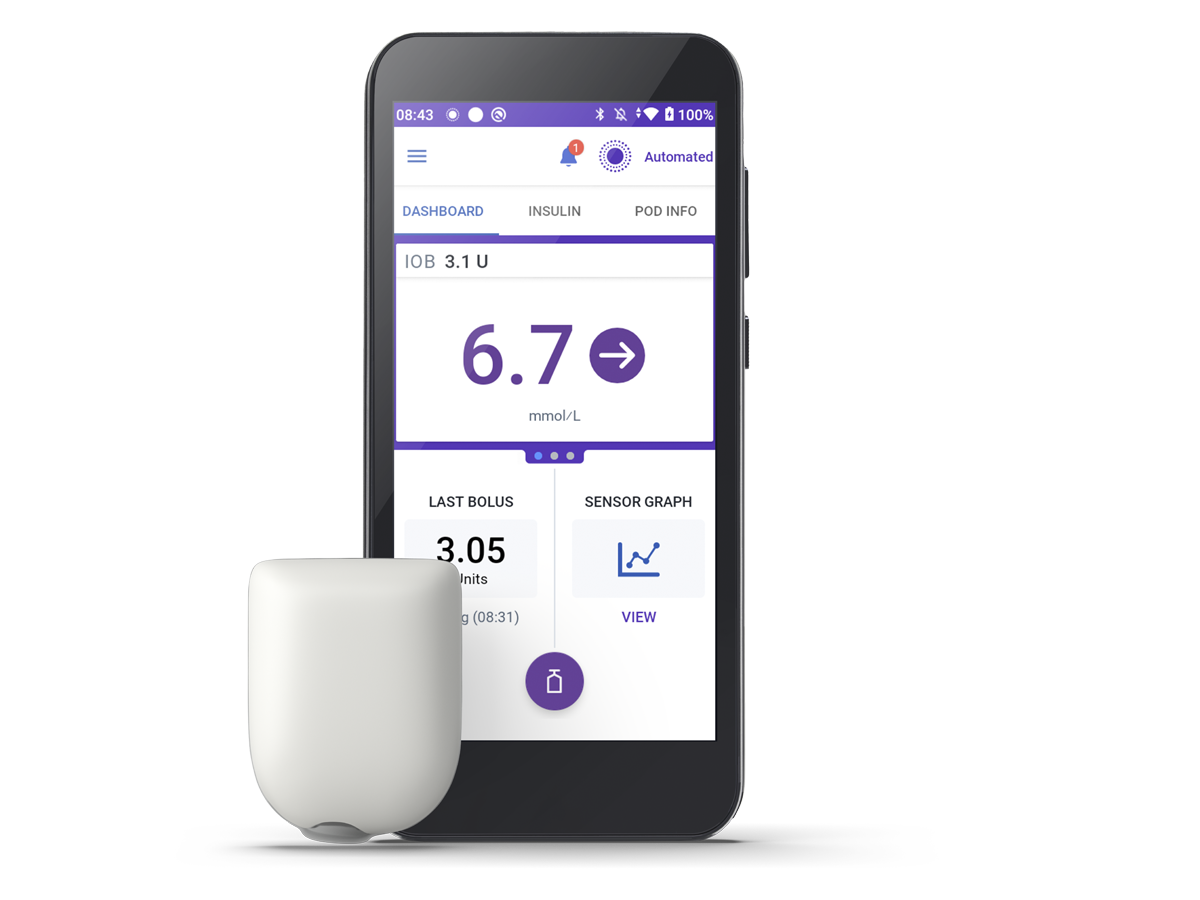

Omnipod 5 Automated Insulin Delivery System
Now compatible with the Dexcom G7 Sensor, as well as the Dexcom G6 Sensor and Freestyle Libre 2 Plus Sensor (launch timings might differ between Sensor’s compatibility)!* Omnipod 5 automatically adjusts insulin every five minutes, helping you to stay in range throughout the day and night1,2.


Omnipod DASH® Insulin Management System
Now compatible with the Dexcom G7 sensor, as well as the Dexcom G6 sensor and Freestyle Libre 2 Plus sensor (launch timings might differ between sensors compatibility)!* Omnipod 5 automatically adjusts insulin every five minutes, helping you to stay in range throughout the day and night1,2. You’re in control with the Omnipod DASH Personal Diabetes Manager. Discover discreet, precise insulin dosing and customisable programmes designed to fit around your lifestyle.
A 37-year road to freedom
Clare was diagnosed with type 1 diabetes over 40 years ago. After decades of multiple daily injections, blood glucose swings, and dangerous lows, Omnipod helped her simplify life with diabetes.
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
Ready to find out more?
Request your free demo Pod** today!
The Pod Experience Kit** contains a real-size, real-weight demo Pod**, without the insulin.
It’s designed to give you an idea of what it feels like to wear a Pod, and get a sense of how discreet it can be.
Talk to an Omnipod Specialist
Our dedicated team can support you with any questions you have about Pod Therapy.
Call on 1800 954 075 or +61 272 084 353 from abroad 9am-6pm, Monday-Friday.
Alternatively, you can request a call at a time that suits you.
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs [adults/adolescents (n= 128; aged 14-70 yrs) children (n=112; aged 6-13.9 yrs)]. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop phase. Mean time 70-180 mg/dL (6AM-12AM) as measured by CGM in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 64.8% vs. 72.5%; 51.5% vs. 64.6%, P<0.0001, respectively. Mean time 70-180 mg/dL (12AM-6AM) as measured by CGM in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 64.3% vs. 78.1%; 55.3% vs. 78.1%, P<0.0001, respectively.
2. Sherr JL, et al. 2022. 45(8):1907–1910. Prospective trial in 80 participants with T1D aged 2 - 5.9 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time 70-180mg/dL (6AM-12AM) as measured by CGM in very young children (2 - 5.9 yrs), ST vs. 3-mo Omnipod 5: 56.9% vs. 63.7%, P<0.0001. Mean time 70-180mg/dL (12AM-6AM) as measured by CGM in very young children (2 - 5.9 yrs), ST vs. 3-mo Omnipod 5: 58.2% vs 81.0%, P<0.0001.