About Omnipod® 5
Meet Omnipod 5
Level up your life with the first and only tubeless automated insulin delivery system that works with Dexcom G6 and G7 Sensors .
- Omnipod 5 has been shown to improve A1C and TIR, day and night1,2
- No multiple daily injections, insulin pump tubing or routine fingersticks†
- Share the load with a system that’s always adjusting, so you don’t have to
- Suitable for people with type 1 diabetes ages 2 years and older
- Integrates with compatible sensors*
Omnipod 5 is an automated insulin delivery (AID) system, which you may know as a hybrid closed loop system. It integrates with Dexcom G6 and G7 Sensors, working day and night, so you can focus more on life and less on diabetes.
Fuel your freedom with a system that delivers automated insulin adjustments every five minutes, responding to predicted glucose levels by automatically increasing, decreasing or pausing insulin delivery depending on whether glucose levels are rising or falling.
At this time, Omnipod 5 is publicly reimbursed in Ontario and Nova Scotia. Insulet is actively working on public reimbursement across different provinces and territories. It is recommended that anyone interested in learning more about the Omnipod 5 System speak with their healthcare provider (HCP) to discuss if they are suitable for the product.
One System. Designed to deliver.
Long gone are the days of choosing between glucose results from an Automated Insulin Delivery System and freedom of a tubeless device.
Demonstrated improved TIR1,2
Demonstrated lower A1C1,2
Adapting to you
3 Simple Parts
To enjoy Automated Insulin Delivery with the Omnipod 5 System
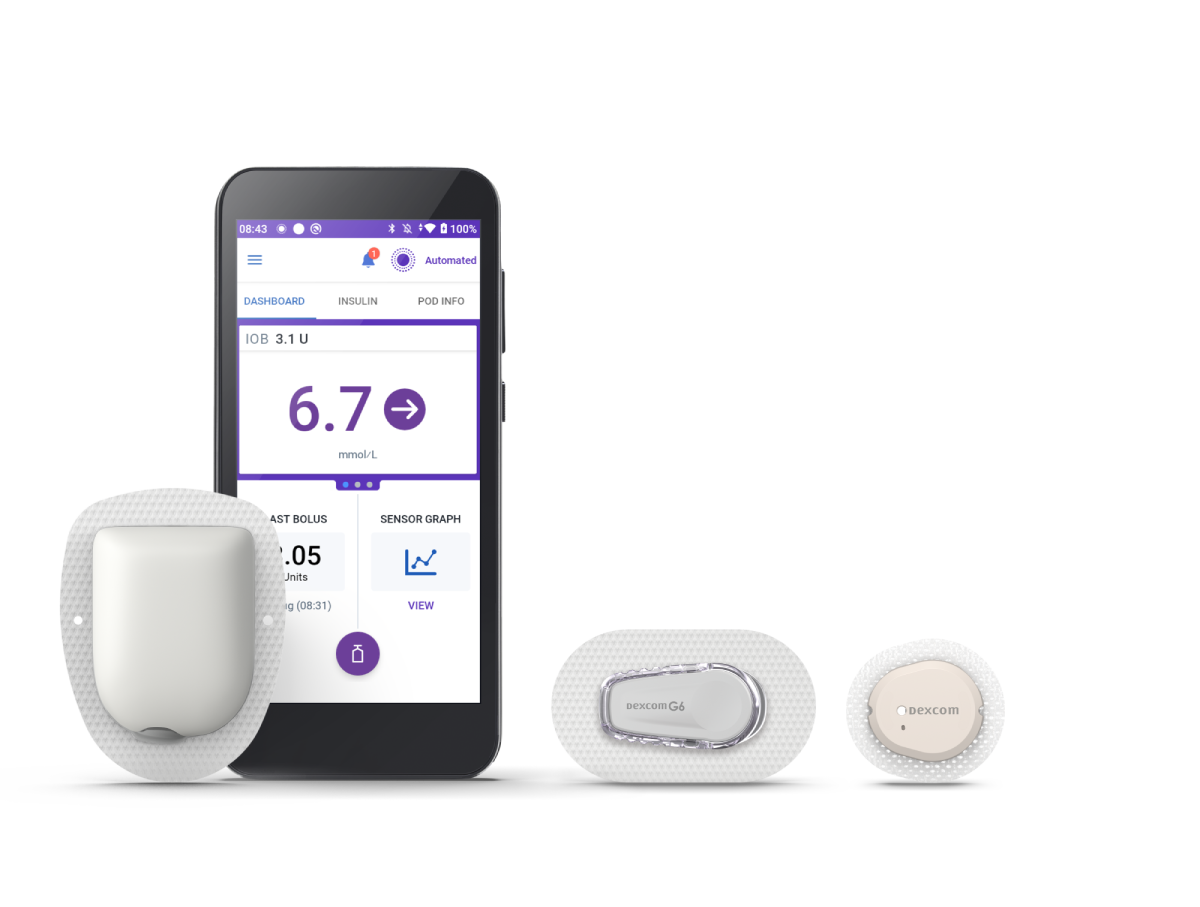

Omnipod 5 Controller
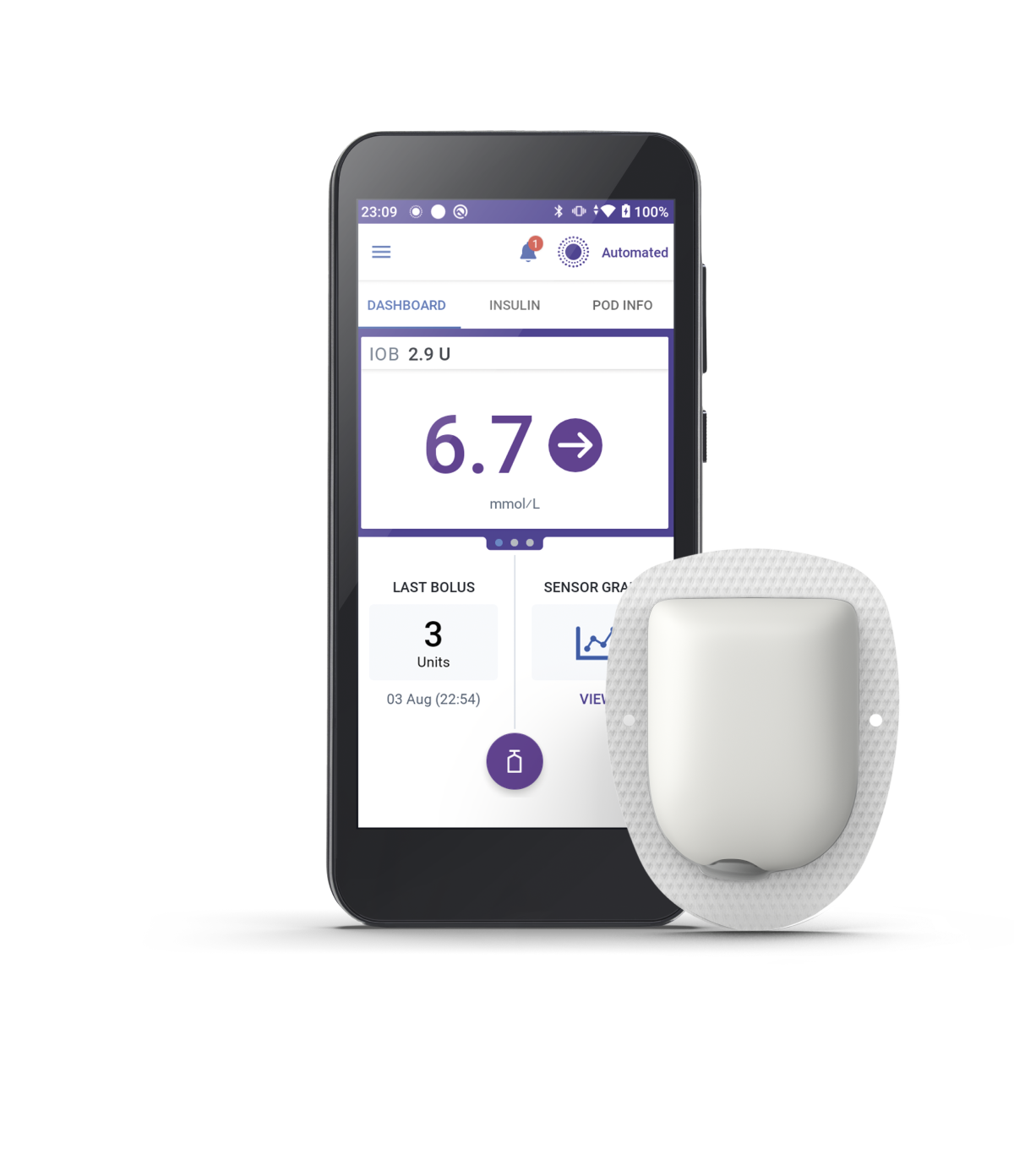
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod's operations using Bluetooth® wireless technology.
Pod
Tubeless, wearable, and waterproof‡, the Pod, with built-in SmartAdjust™ technology, sits on the body and automatically adjusts insulin delivery for up to 3 days (72 hours).
Sensor
Continuously sends glucose values to the Pod, so you can get real-time data§ without the routine fingersticks†. Compatible sensors are sold separately and require a separate prescription. The Pod and sensor need to be in ‘line-of-sight’ to stay in Automated Mode. Placing them on the same side of the body allows for best communication between the devices.
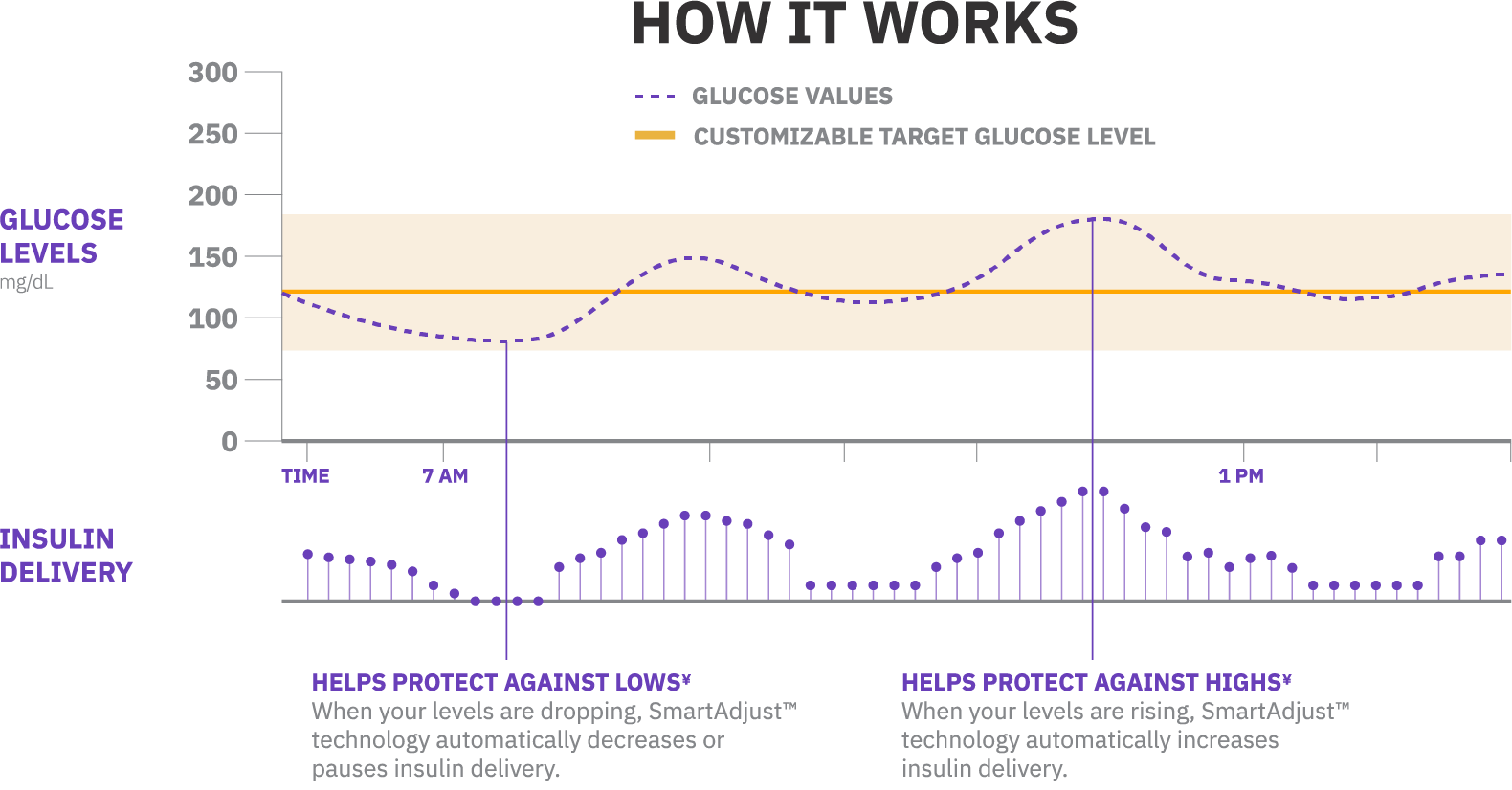

Using our SmartAdjust™ technology, Omnipod 5 and the sensor are in constant communication. This enables automated insulin delivery, freeing you from manually controlling your insulin around the clock. Less work for you, more time in range.1,2
You will still need to bolus for meals and correction doses through the Omnipod 5 Controller, where you monitor and control the Pod's operations using Bluetooth® wireless technology.
Innovation at your fingertips
Omnipod 5 boasts outstanding design and engineering, recognized by the Consumer Electronics Show (CES) Innovation Awards, in the US, in 2022.

Don’t just take our word for it…
Here’s what our Podders have to say about Omnipod 5:
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
I love automated insulin delivery, I can do things a lot more spontaneously. My parents would say it’s been a life-changer for me AND for them.
Mattie G
Mattie G
Omnipod 5 User
With Omnipod 5, I’ve found a new confidence that I didn’t have before… I feel like a new person. Omnipod 5 makes me feel safe.
Morgan M.
Podder since 2009
Ready to Get Started with Omnipod?
Explore your coverage and request a free Pod Experience Kit (PEK)¶ today to try a demo Pod¶ for yourself!
Whether you’re considering Omnipod 5 or Omnipod DASH, our real-size, real-weight demo Pod¶ can help you understand what it feels like to wear the Pod and get a sense of how discreet it can be.
Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription.
†Routine fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
‡The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
§Shah VN, et al. Diabetes Technol and Ther. 2018;20(6).
¶The PEK includes a non-functioning, needle-free Pod which allows you to experience how it feels to wear a Pod. The demo Pod is for demonstrative purposes only and is not able to deliver insulin. This PEK is available only to people with type 1 diabetes who are on a current insulin treatment (e.g., multiple daily injections or insulin pump therapy). People with type 2 diabetes are not eligible to receive a PEK unless they reside in British Columbia. The PEK does not include a physical Omnipod 5 Controller or the Omnipod DASH PDM.
A1C, glycated hemoglobin (HbA1c); TIR, time in range.
1. Brown SA, et al. Diabetes Care. 2021;44(7):1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, p<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, p<0.0001; 2.51% vs. 1.78, p=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, p<0.0001; 2.13% vs. 1.98%, p=0.2545, respectively.
2. Sherr JL, et al. Diabetes Care. 2022;45(8):1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, p=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, p=0.0799.