Is Omnipod® right for me?
93% of Omnipod customers are Satisfied or Very Satisfied with the product
The tubeless, wearable, waterproof* Omnipod has helped simplify thousands of people’s lives. They have built a strong community committed to supporting each other and spreading the word about Pod Therapy. They call themselves Podders.
Why Omnipod?
Omnipod was created out of a father’s mission to free his son from the burden of multiple daily injections. Our mission to simplify life for those living with type 1 diabetes remains.
Omnipod can simplify life with up to 3 days (up to 72 hours) of continuous, tubeless insulin delivery, with no need for multiple daily injections.
- Discreet: The Pod is small enough to be worn under clothing, with no tubing.
- Convenient: Deliver bolus insulin with just a few finger taps using the wireless Controller (Omnipod 5) or Personal Diabetes Manager (Omnipod DASH®).
- Simplified: The built-in Bolus Calculator reduces mealtime math.
- Waterproof*: The Pod can be worn in the shower, swimming pool, or sea.
We have two systems. Our Omnipod DASH System and our next generation automated insulin delivery (AID) system – Omnipod 5. Omnipod 5 is the first and only tubeless automated insulin delivery system that works with compatible sensor brands.†
Pod Therapy is insulin pump therapy, but not as you know it!
Meet our tubeless, wearable, waterproof* Omnipod line-up
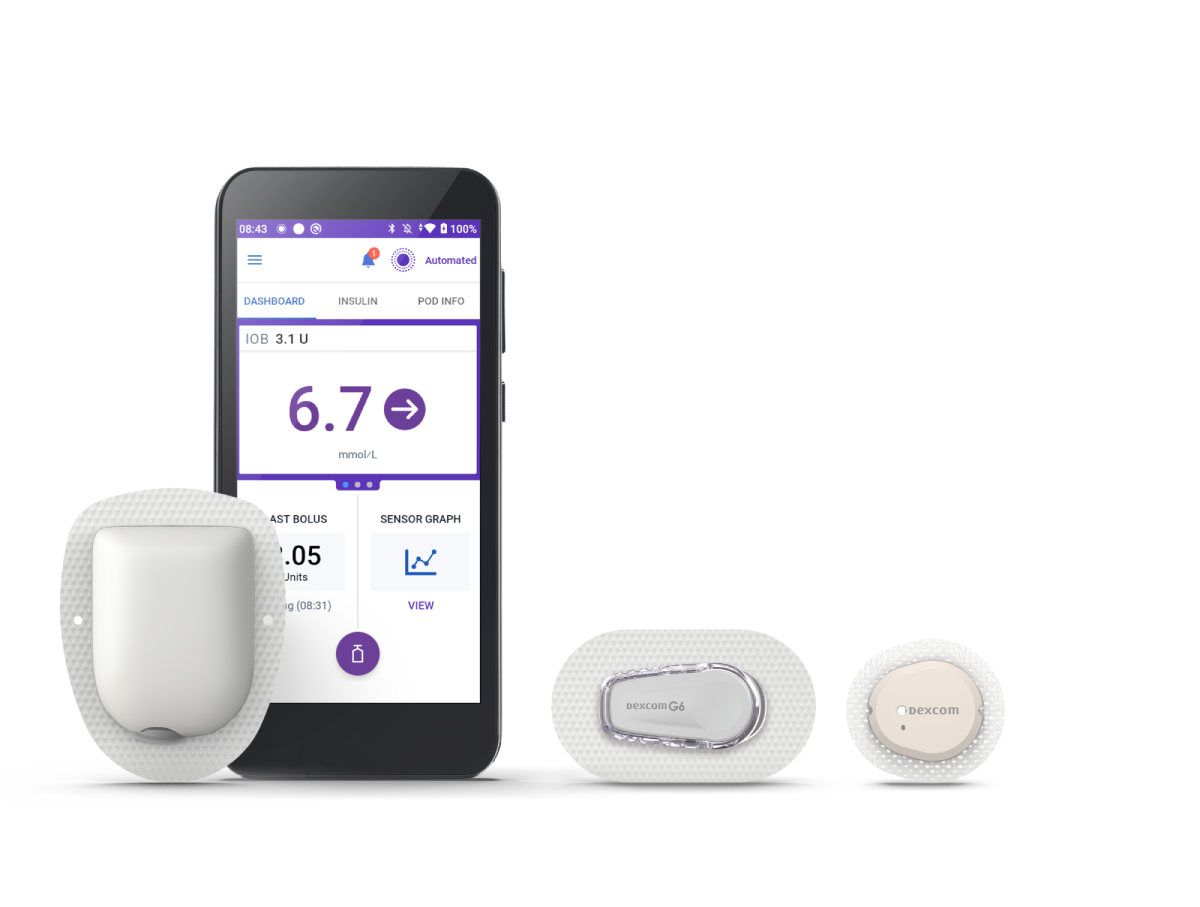

Omnipod 5 Automated Insulin Delivery System
Now compatible with Dexcom G6 and G7 Sensors.† Omnipod 5 automatically adjusts basal insulin every five minutes, helping you to stay in range throughout the day and night.1,2


Omnipod DASH Insulin Management System
You’re in control with the Omnipod DASH Personal Diabetes Manager. Discover discreet, precise insulin dosing and customizable programs designed to fit around your lifestyle.
Ready to find out more about Omnipod?
Order your free demo Pod‡ today!
Experience tubeless freedom by ordering a Pod Experience Kit (PEK)‡, which comes with a real-size, real-weight Demo Pod‡, without the insulin.
It’s designed to give you an idea of what it feels like to wear a Pod and get a sense of how discreet it can be.
Talk to an Omnipod Specialist
Our friendly and helpful team is ready to support you with any questions you may have about Omnipod DASH or Omnipod 5. at 1-855-POD-INFO (1-855-763-4636) or email us at [email protected].
*The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Omnipod 5 Controller and the Omnipod DASH PDM are not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
†Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription.
‡The PEK includes a non-functioning, needle-free Pod which allows you to experience how it feels to wear a Pod. The demo Pod is for demonstrative purposes only and is not able to deliver insulin. This PEK is available only to people with type 1 diabetes who are on a current insulin treatment (e.g., multiple daily injections or insulin pump therapy). People with type 2 diabetes are not eligible to receive a PEK unless they reside in British Columbia. The PEK does not include a physical Omnipod 5 Controller or the Omnipod DASH PDM.
1. Brown SA, et al. Diabetes Care. 2021;44(7):1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Average time in range (70-180mg/dL or 3.9-10 mmol/L) (6AM-12AM) in adults/adolescents and children for standard therapy vs Omnipod 5 = 64.8% vs. 72.5%; 51.5% vs. 64.6%. Average time in range (70-180mg/dL or 3.9-10 mmol/L) (12AM-6AM) in adults/adolescents and children for standard therapy vs Omnipod 5 = 64.3% vs. 78.1%; 55.3% vs. 78.1%.
2. Sherr JL, et al. Diabetes Care. 2022;45(8):1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Average time in range (70-180mg/dL or 3.9-10 mmol/L) (6AM-12AM) in standard therapy vs Omnipod 5 = 56.9% vs. 63.7%. Average time in range (70-180mg/dL or 3.9-10 mmol/L) (12AM-6AM) from CGM in standard therapy vs. Omnipod 5 = 58.2% vs 81.0%.
Dexcom is in the process of discontinuing Dexcom G6 to focus on delivering the next generation of Sensor. Omnipod 5 will continue to support integration with Dexcom G6 for as long as this sensor remains available. For more information, please visit www.dexcom.com or contact Dexcom support.