Omnipod® 5 App for
iPhone Resources
New to Omnipod 5? If you have received an intro kit, go to the Omnipod 5 Setup portal. If you’re still exploring, visit the Omnipod 5 App for iPhone page.
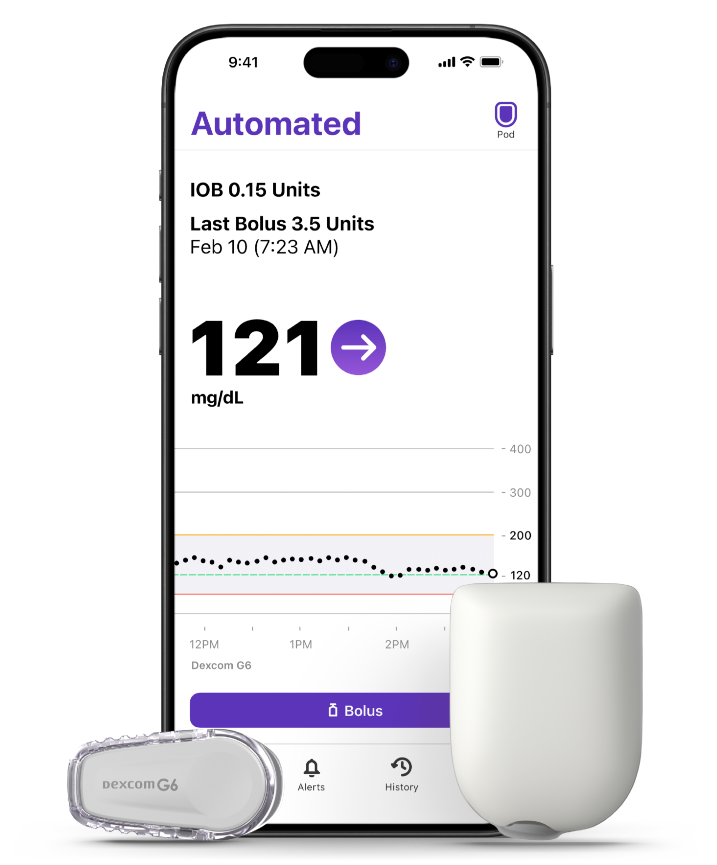

Transitioning to the Omnipod 5 App for iPhone
Your checklist:
You do not need a new prescription to download the App.

Sign in to the App with your Omnipod ID
You’ll need your Omnipod ID and password to log in to the Omnipod 5 App for iPhone. If you’re having trouble, visit Omnipod® 5 FAQs: Omnipod ID and Password.
Transfer your settings
Switching to a new Omnipod 5 device will require you to go through First Time Setup again. Insulin delivery history from previous Pods will be lost when you switch to your new device and adaptivity will start over. Use this guide and video to help transfer your settings:
Important: Confirm your settings with your healthcare provider.
Start a new Pod
To start using Omnipod 5 with your iPhone, you'll need to deactivate and remove your current Pod that is connected to your Controller or Android compatible smartphone. Use your compatible iPhone and Omnipod 5 App to activate a new Pod.
Your Pod should connect to your Dexcom G6 as it does after routine Pod changes. A sensor change is not required.
The Omnipod 5 App for iPhone is compatible with iOS 17, iOS 18
and Dexcom G6.
For more information visit omnipod.com/compatibility.
Videos and User Guides
From System basics to the finer details, we've got you covered with a range of video tutorials and user guides designed for the Omnipod 5 App for iPhone.
For more information, visit the Omnipod 5 for iPhone FAQ page.