Say hello to Omnipod® 5

Meet Omnipod 5
Level up your life with the first and only tubeless automated insulin delivery system that works with Dexcom G6 and G7 Sensors .
- Omnipod 5 has been shown to improve A1C and TIR, day and night1,2
- No multiple daily injections, insulin pump tubing or routine fingersticks†
- Share the load with a system that’s always adjusting, so you don’t have to
- Suitable for people with type 1 diabetes ages 2 years and older
- Integrates with compatible sensors*
Omnipod 5 is an automated insulin delivery (AID) system, which you may know as a hybrid closed loop system. It integrates with Dexcom G6 and G7 Sensors, working day and night, so you can focus more on life and less on diabetes.
Fuel your freedom with a system that delivers automated insulin adjustments every five minutes, responding to predicted glucose levels by automatically increasing, decreasing or pausing insulin delivery depending on whether glucose levels are rising or falling.
At this time, Omnipod 5 is publicly reimbursed in Ontario and Nova Scotia. Insulet is actively working on public reimbursement across different provinces and territories. It is recommended that anyone interested in learning more about the Omnipod 5 System speak with their healthcare provider (HCP) to discuss if they are suitable for the product.
One System. Designed to deliver.
Long gone are the days of choosing between glucose results from an Automated Insulin Delivery System and freedom of a tubeless device.
Demonstrated improved TIR1,2
Demonstrated lower A1C1,2
Adapting to you
3 Simple Parts
To enjoy Automated Insulin Delivery with the Omnipod 5 System
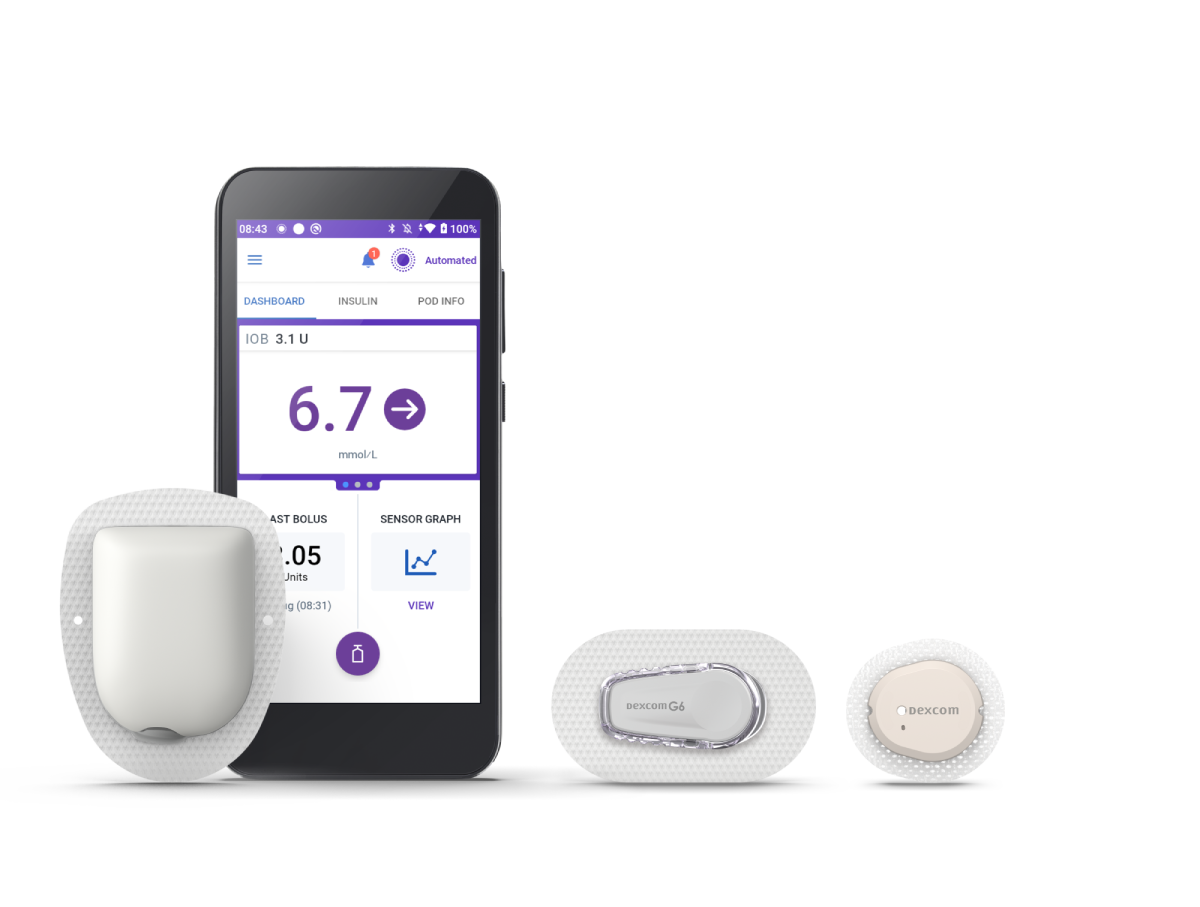

Omnipod 5 Controller
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod's operations using Bluetooth® wireless technology.
Pod
Tubeless, wearable, and waterproof‡, the Pod, with built-in SmartAdjust™ technology, sits on the body and automatically adjusts insulin delivery for up to 3 days (72 hours).
Sensor
Continuously sends glucose values to the Pod, so you can get real-time data§ without the routine fingersticks†. Compatible sensors are sold separately and require a separate prescription. The Pod and sensor need to be in ‘line-of-sight’ to stay in Automated Mode. Placing them on the same side of the body allows for best communication between the devices.
Don’t just take our word for it…
Here’s what our Podders have to say about Omnipod 5:
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
I love automated insulin delivery, I can do things a lot more spontaneously. My parents would say it’s been a life-changer for me AND for them.
Mattie G
Mattie G
Omnipod 5 User
With Omnipod 5, I’ve found a new confidence that I didn’t have before… I feel like a new person. Omnipod 5 makes me feel safe.
Morgan M.
Podder since 2009
Check your Coverage in 3 easy steps.
Step 1. Complete the short Patient Information Form below and hit the SUBMIT button.
Step 2. After submitting the Patient Information Form, you will receive an email with an important link to provide additional information and electronically sign the document. Please remember to complete the form or your request will not move forward.
TIP: If you haven’t received a link within an hour, try checking your junk or spam folder.
Step 3. After you’ve completed and submitted the online form, you’ll be connected with one of our Customer Care Specialists who will help you get started and investigate your coverage.
Interested in Trying Out a Demo Pod?
You can request a free Pod Experience Kit.
Simply complete the Pod Experience Kit (PEK) form below and we will send you a free Pod Experience Kit (PEK)
What's included in the PEK?
- The kit includes a non-functioning & needle-free Demo Pod
- The Demo Pod can be worn almost anywhere you would give yourself an injection for up to 3 days (72 hours) without injecting or receiving insulin
- The Demo Pod gives the wearer an idea of the size, the weight, and how it is worn on the body
- The PEK also comes with a brochure that helps you understand how Omnipod® works.
The PEK does NOT include a Personal Diabetes Manager (PDM)
Please read our Privacy Policy for details on how we manage and protect your information below. We value your privacy and appreciate the trust you place in us by providing it.
Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription.
†Routine fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
‡The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
§Shah VN, et al. Diabetes Technol and Ther. 2018;20(6).
¶The PEK includes a non-functioning, needle-free Pod which allows you to experience how it feels to wear a Pod. The demo Pod is for demonstrative purposes only and is not able to deliver insulin. This PEK is available only to people with type 1 diabetes who are on a current insulin treatment (e.g., multiple daily injections or insulin pump therapy). People with type 2 diabetes are not eligible to receive a PEK unless they reside in British Columbia. The PEK does not include a physical Omnipod 5 Controller or the Omnipod DASH PDM.
A1C, glycated hemoglobin (HbA1c); TIR, time in range.
1. Brown SA, et al. Diabetes Care. 2021;44(7):1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, p<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, p<0.0001; 2.51% vs. 1.78, p=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, p<0.0001; 2.13% vs. 1.98%, p=0.2545, respectively.
2. Sherr JL, et al. Diabetes Care. 2022;45(8):1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, p<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, p<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, p=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, p=0.0799.